the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Technical note: A new online tool for δ18O–temperature conversions
Daniel E. Gaskell
Pincelli M. Hull
The stable-oxygen-isotopic composition of marine carbonates (δ18Oc) is one of the oldest and most widely used paleothermometers. However, interpretation of these data is complicated by the necessity of knowing the δ18O of the source seawater (δ18Ow) from which CaCO3 is precipitated. The effect of local hydrography (the “salinity effect”) is particularly difficult to correct for and may lead to errors of >10 ∘C in sea-surface temperatures if neglected. A variety of methods for calculating δ18Ow have been developed in the literature, but not all are readily accessible to workers. Likewise, temperature estimates are sensitive to a range of other calibration choices (such as calibration species and the inclusion or exclusion of carbonate ion effects), which can require significant effort to intercompare. We present an online tool for δ18O–temperature conversions which provides convenient access to a wide range of calibrations and methods from the literature. Our tool provides a convenient way for workers to examine the effects of alternate calibration and correction procedures on their δ18O-based temperature estimates.
- Article
(2076 KB) - Full-text XML
- BibTeX
- EndNote
The stable-oxygen-isotopic composition of carbonates (δ18Oc) is one of the oldest and most widely used paleothermometers and undergirds a wide variety of paleoceanographic research (for recent reviews, see Pearson, 2012, and Sharp, 2017). Converting δ18Oc to temperature is typically done using an empirical calibration in either a linear form, such as
(Bemis et al., 1998), or in a quadratic form, such as
(McCrea, 1950, as reformulated by Bemis et al., 1998), where T is temperature (in ∘C), δ18Oc is the oxygen isotope composition of the carbonate (as ‰ VPDB, or parts per thousand relative to the Vienna Pee Dee Belemnite standard), and δ18Ow is the oxygen isotope composition of the water in which the carbonate was precipitated (as ‰ VSMOW, or parts per thousand relative to the Vienna Standard Mean Ocean Water standard). Much of the complexity of using δ18O as a paleothermometer arises from the need to know δ18Ow, which may vary both globally as a function of ice volume and locally at the sea surface as a function of regional hydrography (Rohling, 2013). Global variation can be estimated using independent records of sea level, so the global record of deep-water δ18O-based temperatures has been relatively well established (Zachos et al., 2001; Cramer et al., 2009; Westerhold et al., 2020; Rohling et al., 2021; etc.). However, local variations in surface δ18Ow are more difficult to predict, rendering sea-surface temperature (SST) estimates from δ18O less reliable than deep-water temperature estimates. To address this, a variety of methods have been developed in the literature to estimate surface δ18Ow.
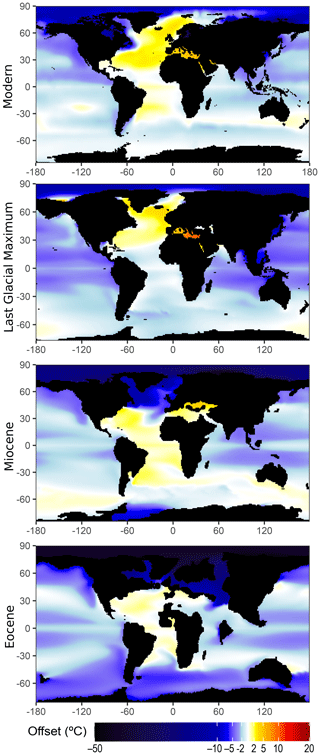
Figure 1Effect of estimating SST using measured/modeled local δ18Ow rather than the latitude-based approximation of Zachos et al. (1994, Eq. 1 therein). Modern: comparison with mean annual δ18Ow<50 m depth (after LeGrande and Schmidt, 2006). Last Glacial Maximum (LGM): comparison with inferred annual surface δ18Ow at the LGM (Tierney et al., 2020). Miocene: comparison with CESMv1.2_CAM5 model run at 400 ppm CO2 with Miocene paleogeography (Gaskell et al., 2022). Eocene: comparison with CESM_1.2_CAM5 model run at 6 times the preindustrial CO2 with Eocene paleogeography (Zhu et al., 2020). Temperatures are calculated assuming a slope of 4.80 ∘C ‰−1 (Bemis et al., 1998).
Since modern surface δ18Ow broadly covaries with latitude, a common approach has been to apply the modern latitudinal variation to a sample's paleolatitude (typically using the relationship fit from Southern Ocean data in Eq. 1 of Zachos et al., 1994, or more recently the updated method of Hollis et al., 2019). However, this approach performs particularly poorly in the North Atlantic and other high northern latitudes, where local δ18Ow can deviate significantly from the latitudinal mean (Fig. 1; Zachos et al., 1994; Gaskell et al., 2022; see also generally Tindall et al., 2010). It also assumes that the latitudinal gradient in δ18Ow has not changed through time, which is contradicted by modeling. In warmer climates with an altered hydrological cycle, models predict that regional salinity contrasts should change due to alterations in the local ratio of evaporation to precipitation (Richter and Xie, 2010; Singh et al., 2016), with an analogous effect on δ18Ow (Zhou et al., 2008; Tindall et al., 2010; Roberts et al., 2011; Zhu et al., 2020). In particularly extreme cases such as the Eocene, the theoretical difference between modern latitude-derived δ18Ow (after Zachos et al., 1994, Eq. 1 therein) and modeled local δ18Ow at 6 times the preindustrial pCO2 (Zhu et al., 2020) yields a mean temperature error of 5 ∘C in the Southern Ocean (60–90∘ S) or an astonishing mean temperature error of 41 ∘C above the Arctic Circle (66.5–90∘ N; Fig. 1).
An alternative approach is to obtain δ18Ow more or less directly from isotope-enabled climate models (Zhou et al., 2008; Roberts et al., 2011; Gaskell et al., 2022). Several approaches have been adopted: drawing local δ18Ow directly from model output (Roberts et al., 2011); using modeled zonal mean δ18Ow for a particular paleolatitude (Zhou et al., 2008); using models as input to fit a generalized equation for predicting δ18Ow from latitude and bottom-water temperature (Gaskell et al., 2022 Eq. S9); or, recently, a generalized method which uses bottom-water temperature to interpolate local δ18Ow between models run at different pCO2 (Gaskell et al., 2022). While some authors have avoided these approaches altogether due to the uncertainty in modeled δ18Ow (e.g., Hollis et al., 2012) or the possibility of introducing circularity into data–model comparisons (e.g., Hollis et al., 2019), model-derived δ18Ow clearly captures information lost by simpler approaches and is therefore appropriate for some use cases (Roberts et al., 2011).
Here, a new online tool for δ18O temperature conversion is presented which automates a range of methods for δ18Ow reconstruction and correction from the literature, improving the accessibility of advanced methods to workers generating δ18Oc data.
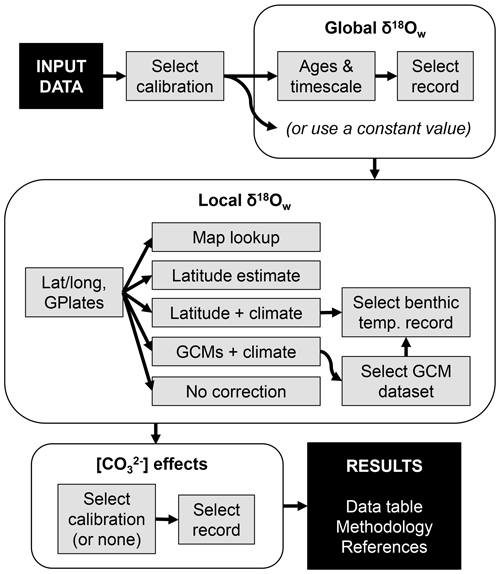
We present a new online tool for performing δ18Oc–temperature conversions which automates a range of methods from the literature. This tool is available at https://research.peabody.yale.edu/d180/ (last access: 7 June 2023). The general workflow for using the tool is summarized in Fig. 2; details on the methodology and reasoning behind each option are given below.
2.1 δ18Oc–temperature calibration
After manually entering or uploading a data sheet of δ18Oc measurements in .csv format, users may select from 1 of 59 different calibrations from the literature (Bemis et al., 1998; Böhm et al., 2000; Bouvier-Soumagnac and Duplessy, 1985; Duplessy et al., 2002; Epstein et al., 1953; Erez and Luz, 1983; Farmer et al., 2007; Geffen, 2012; Godiksen et al., 2010; Grossman and Ku, 1986; Høie et al., 2004; Juillet-Leclerc and Schmidt, 2001; Kim and O'Neil, 1997; Kim et al., 2007; Lynch-Stieglitz et al., 1999; Malevich et al., 2019; Marchitto et al., 2014; McCrea, 1950; Mulitza et al., 2003; O'Neil et al., 1969; Patterson et al., 1993; Reynaud-Vaganay et al., 1999; Rosenheim et al., 2009; Shackleton, 1974; Storm-Suke et al., 2007; Thorrold et al., 1997; Tremaine et al., 2011; White et al., 1999; Willmes et al., 2019). All data are expressed with δ18Oc in units of ‰ VPDB and δ18Ow in units of ‰ VSMOW, with any standard interconversions expected by the chosen calibration performed automatically. Standard interconversion is notably inconsistent in the literature, with many paleoceanographic papers employing the relationship ‰ (Hut, 1987), while many geochemical papers employ the incompatible relationship ‰ (Brand et al., 2014). The former is actually the isotopic offset between the related VPDB-CO2 and VSMOW-CO2 scales, but the difference is unimportant so long as all data are treated in the manner the calibration expects, as our tool ensures. A full list of included calibrations and standard conversions is given in Tables 1–3.
Table 1Linear and quadratic δ18O: temperature calibrations of the form , where , with the given VSMOW conversion factor first added to δ18Ow to convert VSMOW into the format expected by the calibration.
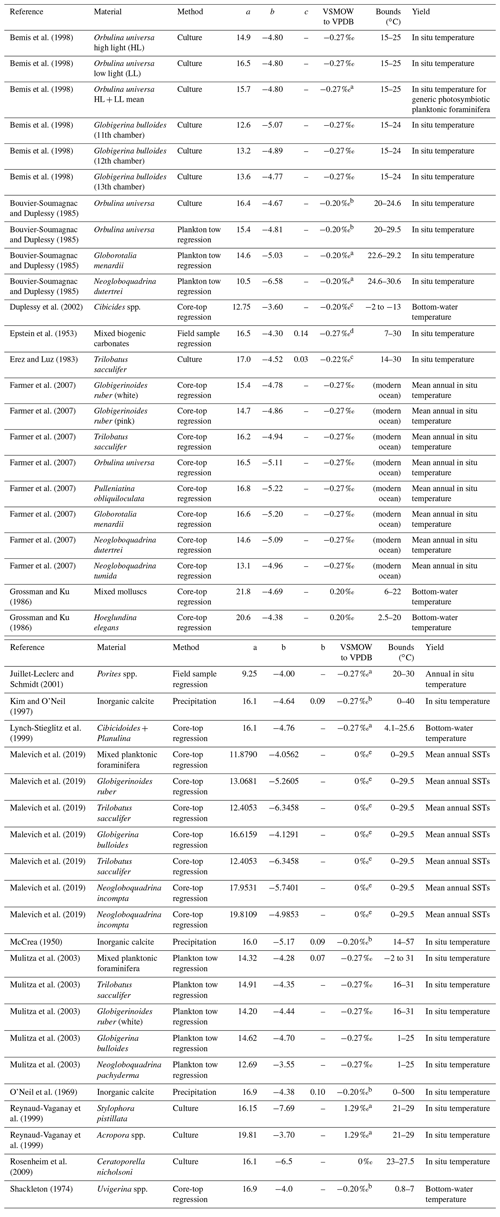
a Rearranged by this work; b reformulated by Bemis et al. (1998); c reformulated by Mulitza et al. (2003); d reformulated by Bemis et al. (1998), with VSMOW correction after Grossman (2012); e reformulated in this work by extracting the linear coefficients from the Bayesian posterior values.
Table 2Logarithmic δ18O: temperature calibrations of the form , where TK is temperature (in kelvin), and α is the fractionation factor . The temperature solution for this form (in ∘C) is , where the relationship δ18OVPDB=0.97001 δ18OVSMOW−29.99 (Brand et al., 2014) is first applied to convert δ18Oc from VPDB to VSMOW or to convert δ18Ow from VSMOW to VPDB, as required by the calibration. (The value requiring conversion is indicated in the “Convert which” column.)
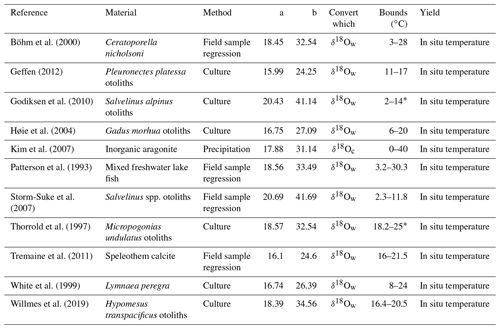
* Reformulated by Willmes et al. (2019).
Table 3δ18O: temperature calibrations in other forms. ; the VSMOW–VPDB conversion is included in the equations below, with no further conversion required.
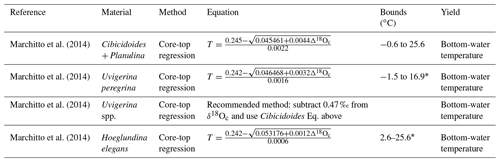
* Rearranged by this work.
Where applicable, we use the standardized reformulations of Bemis et al. (1998) and Willmes et al. (2019) or exact algebraic rearrangements of the original equations. For the bayfox core-top calibrations of Malevich et al. (2019), the standard bayfox tool re-fits the calibration coefficients with every run. Since this is computationally expensive, we instead use the linear calibration coefficients fit by runs of the R package bayfoxr 0.0.1 directly in linear functions of the form of Eq. (1) (see Table 1). These yield results equivalent to the full fitting process within numerical error (mean residual = ±0.02 ∘C, identical to the mean scatter between replicates of the full bayfox fit).
2.2 Global δ18Ow estimation
Users may specify global δ18Ow manually or choose to draw δ18Ow by sample age from 12 different time series of global δ18Ow from the literature (from Cramer et al., 2011; Henkes et al., 2018; Meckler et al., 2022; Miller et al., 2020; Modestou et al., 2020; Rohling et al., 2021; and Veizer and Prokoph, 2015). These records are typically constructed by assuming that the benthic δ18O record reflects a combination of temperature and ice volume and then subtracting out an independent record of temperature (e.g., using -based bottom-water temperatures; Cramer et al., 2011) or ice volume (e.g., using a multi-proxy sea level reconstruction; Rohling et al., 2021) to determine the residual δ18Ow. Which global δ18Ow record is most realistic remains a contentious topic in the literature, with sea-level and -based records (e.g., Cramer et al., 2011; Rohling et al., 2021) predicting up to ∼1 ‰ lower δ18Ow for much of the Cenozoic than records based on clumped isotope paleothermometry (Meckler et al., 2022; see also Agterhuis et al., 2022). We provide both classes of record here for comparison by the user.
Records are mapped to the user data's ages by linear interpolation. The Δ47-based δ18Ow records of Meckler et al. (2022) included in our tool were generated by interpolating the authors' original results to 0.1 Myr resolution using the Monte Carlo LOESS method and parameters described in the original publication (Meckler et al., 2022).
All built-in δ18Ow and temperature records are internally converted to four different timescales, so the user can select the timescale consistent with their data: GTS2004 (Gradstein et al., 2005), GTS2012 (Gradstein et al., 2012), GTS2016 (Ogg et al., 2016), and GTS2020 (Gradstein et al., 2020). These timescale conversions are performed by linear interpolation between magnetochron boundaries; dataset files can be found on the project GitHub.
2.3 Local δ18Ow estimation
The user may select a method for estimating local δ18Ow. These are as follows: performing no local correction, using modern δ18Ow from each sample's location and a specified depth (after LeGrande and Schmidt, 2006), using reconstructed Late Holocene or Last Glacial Maximum surface δ18Ow from each sample's location (model output from Tierney et al., 2020), using δ18Ow estimated from latitude alone (after Eq. 1 in Zachos et al., 1994, or the method of Hollis et al., 2019), using δ18Ow estimated from latitude and bottom-water temperature (after Gaskell et al., 2022 Eq. S9), or using δ18Ow estimated from isotope-enabled climate models (GCMs; after the method of Gaskell et al., 2022, presently provided using the datasets of Miocene and Eocene paleogeography used in that publication).
For methods which draw from an existing dataset of δ18Ow, the user may specify a number of degrees latitude/longitude or great-circle radius to average over in order to capture a regional mean when the exact paleocoordinates or local hydrography may not be known. To help determine site locations at the time of deposition, an option is also provided to automatically perform paleocoordinate rotations using the GPlates Web Service (Müller et al., 2018). Ages passed to GPlates are rounded to the nearest 100 ka to reduce the number of calls to the web service.
Our tool does not currently implement any automated consideration of seasonal variation in local δ18Ow, as this is generally treated as negligible by standard methodologies or implicitly baked into the calibration by calibrating against mean annual temperatures and δ18Ow values (e.g., Malevich et al., 2019).
2.4 Carbonate chemistry effects
Because δ18Oc is known to vary with aqueous carbonate chemistry (the “carbonate ion effect”; Spero et al., 1997; Bijma et al., 1999; Ziveri et al., 2012), users may also specify a carbonate ion correction factor. This is performed by adjusting δ18Oc with the linear relationship
where δ18Oc is the uncorrected oxygen isotope composition of the carbonate, δ18Oc′ is the corrected oxygen isotope composition of the carbonate, s is the selected slope of the effect (in ‰ VPDB per µmol L−1 CO), and [CO] is the concentration of carbonate ion in solution (in µmol kg−1). This relationship yields no correction when [CO] = 200 µmol kg−1, an approximation of the mean modern surface value (after the long-term record of Zeebe and Tyrrell, 2019). The user may specify [CO] manually or select a published long-term record of [CO] (Tyrrell and Zeebe, 2004; Zeebe and Tyrrell, 2019).
2.5 Tool output
On completion, the tool presents a formatted table of the resulting temperatures, along with any intermediate values (such as estimated δ18Ow) which were required to generate them. Any rows with potential errors (e.g., paleocoordinates which do not yield a valid δ18Ow estimate or temperatures which exceed the data range of the calibration) are highlighted in color and flagged with warning text, which appears in an adjacent column. For reference, a short summary of methods is also generated, including relevant equations and a complete bibliography of citations in both text and BibTeX formats for the methods employed in each run.
It should be noted that, while the tool automates the process of applying a given calibration method, the user is still responsible for pre-screening their data for diagenetic alteration or other external biases. For example, use of δ18O data from foraminifera must consider factors such as diagenetic recrystallization, depth habitat, shell size, and the presence of gametogenic calcite (for a review, see Pearson, 2012).
Our tool provides a convenient way for workers to perform δ18O–temperature conversions and explore the sensitivity of their results to different calibrations, corrections, and δ18Ow reconstruction methods by successively trying different options in the interface. By allowing data generators to rapidly generate multiple temperature estimates for their records with different underlying assumptions, our tool allows workers to quickly understand and quantify the effects of different assumptions on the resulting temperature estimates.
An online version of the most current release of our tool is maintained at https://research.peabody.yale.edu/d180/index.html (Gaskell and Hull, 2023a). Source code (JavaScript and PHP) is available from the project's GitHub repository at https://github.com/danielgaskell/d18Oconverter (last access: 13 June 2023) (https://doi.org/10.5281/zenodo.7946599, Gaskell and Hull, 2023b).
All referenced datasets are included in the source repository for the tool (https://doi.org/10.5281/zenodo.7946599; Gaskell and Hull, 2023b).
DEG and PMH conceptualized the tool. DEG wrote the software. DEG and PMH contributed to the manuscript writing.
The contact author has declared that neither of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank Matthew Huber, Charlotte L. O'Brien, Gordon N. Inglis, R. Paul Acosta, and Christopher J. Poulsen for discussions which contributed to the design of this tool and associated methodologies. We thank Brett Metcalfe and an anonymous referee for their constructive comments, which improved the manuscript. We acknowledge institutional support from the Yale Peabody Museum and assistance from Nelson Rios in hosting the tool.
This research has been supported by the National Science Foundation (grant no. 1702851).
This paper was edited by Marit-Solveig Seidenkrantz and reviewed by Brett Metcalfe and one anonymous referee.
Agterhuis, T., Ziegler, M., de Winter, N. J., and Lourens, L. J.: Warm deep-sea temperatures across Eocene Thermal Maximum 2 from clumped isotope thermometry, Commun. Earth Environ., 3, 1–9, https://doi.org/10.1038/s43247-022-00350-8, 2022.
Bemis, B. E., Spero, H. J., Bijma, J., and Lea, D. W.: Reevaluation of the oxygen isotopic composition of planktonic foraminifera: Experimental results and revised paleotemperature equations, Paleoceanography, 13, 150–160, https://doi.org/10.1029/98PA00070, 1998.
Bijma, J., Spero, H. J., and Lea, D. W.: Reassessing Foraminiferal Stable Isotope Geochemistry: Impact of the Oceanic Carbonate System (Experimental Results), in: Use of Proxies in Paleoceanography, edited by: Fischer, D. G. and Wefer, P. D. G., Springer, Berlin, Heidelberg, 489–512, https://doi.org/10.1007/978-3-642-58646-0_20, 1999.
Böhm, F., Joachimski, M. M., Dullo, W.-C., Eisenhauer, A., Lehnert, H., Reitner, J., and Wörheide, G.: Oxygen isotope fractionation in marine aragonite of coralline sponges, Geochim. Cosmochim. Ac., 64, 1695–1703, https://doi.org/10.1016/S0016-7037(99)00408-1, 2000.
Bouvier-Soumagnac, Y. and Duplessy, J.-C.: Carbon and oxygen isotopic composition of planktonic foraminifera from laboratory culture, plankton tows and Recent sediment; implications for the reconstruction of paleoclimatic conditions and of the global carbon cycle, J. Foraminif. Res., 15, 302–320, https://doi.org/10.2113/gsjfr.15.4.302, 1985.
Brand, W. A., Coplen, T. B., Vogl, J., Rosner, M., and Prohaska, T.: Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report), Pure Appl. Chem., 86, 425–467, 2014.
Cramer, B. S., Toggweiler, J. R., Wright, J. D., Katz, M. E., and Miller, K. G.: Ocean overturning since the Late Cretaceous: Inferences from a new benthic foraminiferal isotope compilation, Paleoceanography, 24, PA4216, https://doi.org/10.1029/2008PA001683, 2009.
Cramer, B. S., Miller, K. G., Barrett, P. J., and Wright, J. D.: Late Cretaceous–Neogene trends in deep ocean temperature and continental ice volume: Reconciling records of benthic foraminiferal geochemistry (δ18O and ) with sea level history, J. Geophys. Res.-Oceans, 116, C12023, https://doi.org/10.1029/2011JC007255, 2011.
Duplessy, J.-C., Labeyrie, L., and Waelbroeck, C.: Constraints on the ocean oxygen isotopic enrichment between the Last Glacial Maximum and the Holocene: Paleoceanographic implications, Quaternary Sci. Rev., 21, 315–330, https://doi.org/10.1016/S0277-3791(01)00107-X, 2002.
Epstein, S., Buchsbaum, R., Lowenstam, H. A., and Urey, H. C.: Revised carbonate-water isotopic temperature scale, Geol. Soc. Am. Bull., 64, 1315–1326, 1953.
Erez, J. and Luz, B.: Experimental paleotemperature equation for planktonic foraminifera, Geochim. Cosmochim. Ac., 47, 1025–1031, https://doi.org/10.1016/0016-7037(83)90232-6, 1983.
Farmer, E. C., Kaplan, A., de Menocal, P. B., and Lynch-Stieglitz, J.: Corroborating ecological depth preferences of planktonic foraminifera in the tropical Atlantic with the stable oxygen isotope ratios of core top specimens, Paleoceanography, 22, PA3205, https://doi.org/10.1029/2006PA001361, 2007.
Gaskell, D. E. and Hull, P. M.: δ18O to temperature converter, Yale Peabody Museum, https://research.peabody.yale.edu/d180/index.html (last access: 7 June 2023), 2023a.
Gaskell, D. E. and Hull, P. M.: δ18O to temperature converter, Zenodo [code and data set], https://doi.org/10.5281/zenodo.7946599, 2023b.
Gaskell, D. E., Huber, M., O'Brien, C. L., Inglis, G. N., Acosta, R. P., Poulsen, C. J., and Hull, P. M.: The latitudinal temperature gradient and its climate dependence as inferred from foraminiferal δ18O over the past 95 million years, P. Natl. Acad. Sci. USA, 119, e2111332119, https://doi.org/10.1073/pnas.2111332119, 2022.
Geffen, A. J.: Otolith oxygen and carbon stable isotopes in wild and laboratory-reared plaice (Pleuronectes platessa), Environ. Biol. Fish., 95, 419–430, https://doi.org/10.1007/s10641-012-0033-2, 2012.
Godiksen, J. A., Svenning, M.-A., Dempson, J. B., Marttila, M., Storm-Suke, A., and Power, M.: Development of a species-specific fractionation equation for Arctic charr (Salvelinus alpinus (L.)): an experimental approach, Hydrobiologia, 650, 67–77, https://doi.org/10.1007/s10750-009-0056-7, 2010.
Gradstein, F. M., Ogg, J. G., and Smith, A. G. (Eds.): A Geologic Time Scale 2004, Cambridge University Press, Cambridge, https://doi.org/10.1017/CBO9780511536045, 2005.
Gradstein, F. M., Ogg, J. G., Schmitz, M. D., and Ogg, G. M. (Eds.): The Geologic Time Scale, Elsevier, https://doi.org/10.1016/C2011-1-08249-8, 2012.
Gradstein, F. M., Ogg, J. G., Schmitz, M. D., and Ogg, G. M.: Geologic Time Scale 2020, Elsevier, https://doi.org/10.1016/C2020-1-02369-3, 2020.
Grossman, E. L.: Chapter 10 – Oxygen Isotope Stratigraphy, in: The Geologic Time Scale, edited by: Gradstein, F. M., Ogg, J. G., Schmitz, M. D., and Ogg, G. M., Elsevier, Boston, 181–206, https://doi.org/10.1016/B978-0-444-59425-9.00010-X, 2012.
Grossman, E. L. and Ku, T.-L.: Oxygen and carbon isotope fractionation in biogenic aragonite: Temperature effects, Chem. Geol., 59, 59–74, https://doi.org/10.1016/0168-9622(86)90057-6, 1986.
Henkes, G. A., Passey, B. H., Grossman, E. L., Shenton, B. J., Yancey, T. E., and Pérez-Huerta, A.: Temperature evolution and the oxygen isotope composition of Phanerozoic oceans from carbonate clumped isotope thermometry, Earth Planet. Sc. Lett., 490, 40–50, https://doi.org/10.1016/j.epsl.2018.02.001, 2018.
Høie, H., Otterlei, E., and Folkvord, A.: Temperature-dependent fractionation of stable oxygen isotopes in otoliths of juvenile cod (Gadus morhua L.), ICES J. Mar. Sci., 61, 243–251, https://doi.org/10.1016/j.icesjms.2003.11.006, 2004.
Hollis, C. J., Taylor, K. W. R., Handley, L., Pancost, R. D., Huber, M., Creech, J. B., Hines, B. R., Crouch, E. M., Morgans, H. E. G., Crampton, J. S., Gibbs, S., Pearson, P. N., and Zachos, J. C.: Early Paleogene temperature history of the Southwest Pacific Ocean: Reconciling proxies and models, Earth Planet. Sc. Lett., 349–350, 53–66, https://doi.org/10.1016/j.epsl.2012.06.024, 2012.
Hollis, C. J., Dunkley Jones, T., Anagnostou, E., Bijl, P. K., Cramwinckel, M. J., Cui, Y., Dickens, G. R., Edgar, K. M., Eley, Y., Evans, D., Foster, G. L., Frieling, J., Inglis, G. N., Kennedy, E. M., Kozdon, R., Lauretano, V., Lear, C. H., Littler, K., Lourens, L., Meckler, A. N., Naafs, B. D. A., Pälike, H., Pancost, R. D., Pearson, P. N., Röhl, U., Royer, D. L., Salzmann, U., Schubert, B. A., Seebeck, H., Sluijs, A., Speijer, R. P., Stassen, P., Tierney, J., Tripati, A., Wade, B., Westerhold, T., Witkowski, C., Zachos, J. C., Zhang, Y. G., Huber, M., and Lunt, D. J.: The DeepMIP contribution to PMIP4: methodologies for selection, compilation and analysis of latest Paleocene and early Eocene climate proxy data, incorporating version 0.1 of the DeepMIP database, Geosci. Model Dev., 12, 3149–3206, https://doi.org/10.5194/gmd-12-3149-2019, 2019.
Hut, G.: Consultants' group meeting on stable isotope reference samples for geochemical and hydrological investigations, International Atomic Energy Agency, Vienna, Austria, https://inis.iaea.org/search/search.aspx?orig_q=RN:18075746 (last access: 7 June 2023), 1987.
Juillet-Leclerc, A. and Schmidt, G.: A calibration of the oxygen isotope paleothermometer of coral aragonite from Porites, Geophys. Res. Lett., 28, 4135–4138, https://doi.org/10.1029/2000GL012538, 2001.
Kim, S.-T. and O'Neil, J. R.: Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates, Geochim. Cosmochim. Ac., 61, 3461–3475, https://doi.org/10.1016/S0016-7037(97)00169-5, 1997.
Kim, S.-T., O'Neil, J. R., Hillaire-Marcel, C., and Mucci, A.: Oxygen isotope fractionation between synthetic aragonite and water: Influence of temperature and Mg2+ concentration, Geochim. Cosmochim. Ac., 71, 4704–4715, https://doi.org/10.1016/j.gca.2007.04.019, 2007.
LeGrande, A. N. and Schmidt, G. A.: Global gridded data set of the oxygen isotopic composition in seawater, Geophys. Res. Lett., 33, L12604, https://doi.org/10.1029/2006GL026011, 2006.
Lynch-Stieglitz, J., Curry, W. B., and Slowey, N.: A geostrophic transport estimate for the Florida Current from the oxygen isotope composition of benthic foraminifera, Paleoceanography, 14, 360–373, https://doi.org/10.1029/1999PA900001, 1999.
Malevich, S. B., Vetter, L., and Tierney, J. E.: Global Core Top Calibration of δ18O in Planktic Foraminifera to Sea Surface Temperature, Paleoceanogr. Paleoclimatol., 34, 1292–1315, https://doi.org/10.1029/2019PA003576, 2019.
Marchitto, T. M., Curry, W. B., Lynch-Stieglitz, J., Bryan, S. P., Cobb, K. M., and Lund, D. C.: Improved oxygen isotope temperature calibrations for cosmopolitan benthic foraminifera, Geochim. Cosmochim. Ac., 130, 1–11, https://doi.org/10.1016/j.gca.2013.12.034, 2014.
McCrea, J. M.: On the Isotopic Chemistry of Carbonates and a Paleotemperature Scale, J. Chem. Phys., 18, 849–857, https://doi.org/10.1063/1.1747785, 1950.
Meckler, A. N., Sexton, P. F., Piasecki, A. M., Leutert, T. J., Marquardt, J., Ziegler, M., Agterhuis, T., Lourens, L. J., Rae, J. W. B., Barnet, J., Tripati, A., and Bernasconi, S. M.: Cenozoic evolution of deep ocean temperature from clumped isotope thermometry, Science, 377, 86–90, https://doi.org/10.1126/science.abk0604, 2022.
Miller, K. G., Browning, J. V., Schmelz, W. J., Kopp, R. E., Mountain, G. S., and Wright, J. D.: Cenozoic sea-level and cryospheric evolution from deep-sea geochemical and continental margin records, Sci. Adv., 6, eaaz1346, https://doi.org/10.1126/sciadv.aaz1346, 2020.
Modestou, S. E., Leutert, T. J., Fernandez, A., Lear, C. H., and Meckler, A. N.: Warm Middle Miocene Indian Ocean Bottom Water Temperatures: Comparison of Clumped Isotope and -Based Estimates, Paleoceanogr. Paleoclimatol., 35, e2020PA003927, https://doi.org/10.1029/2020PA003927, 2020.
Mulitza, S., Boltovskoy, D., Donner, B., Meggers, H., Paul, A., and Wefer, G.: Temperature:δ18O relationships of planktonic foraminifera collected from surface waters, Palaeogeogr. Palaeoclimatol. Palaeoecol., 202, 143–152, https://doi.org/10.1016/S0031-0182(03)00633-3, 2003.
Müller, R. D., Cannon, J., Qin, X., Watson, R. J., Gurnis, M., Williams, S., Pfaffelmoser, T., Seton, M., Russell, S. H. J., and Zahirovic, S.: GPlates: Building a Virtual Earth Through Deep Time, Geochem. Geophy. Geosy., 19, 2243–2261, https://doi.org/10.1029/2018GC007584, 2018.
Ogg, J. G., Ogg, G. M., and Gradstein, F. M.: A Concise Geologic Time Scale, Elsevier, https://doi.org/10.1016/C2009-0-64442-1, 2016.
O'Neil, J. R., Clayton, R. N., and Mayeda, T. K.: Oxygen Isotope Fractionation in Divalent Metal Carbonates, J. Chem. Phys., 51, 5547–5558, https://doi.org/10.1063/1.1671982, 1969.
Patterson, W. P., Smith, G. R., and Lohmann, K. C.: Continental Paleothermometry and Seasonality Using the Isotopic Composition of Aragonitic Otoliths of Freshwater Fishes, in: Climate Change in Continental Isotopic Records, AGU – American Geophysical Union, 191–202, https://doi.org/10.1029/GM078p0191, 1993.
Pearson, P. N.: Oxygen Isotopes in Foraminifera: Overview and Historical Review, The Paleontological Society Papers, 18, 1–38, https://doi.org/10.1017/S1089332600002539, 2012.
Reynaud-Vaganay, S., Gattuso, J.-P., Cuif, J.-P., Jaubert, J., and Juillet-Leclerc, A.: A novel culture technique for scleractinian corals: application to investigate changes in skeletal δ18O as a function of temperature, Mar. Ecol. Prog. Ser., 180, 121–130, https://doi.org/10.3354/meps180121, 1999.
Richter, I. and Xie, S.-P.: Moisture transport from the Atlantic to the Pacific basin and its response to North Atlantic cooling and global warming, Clim. Dynam., 35, 551–566, https://doi.org/10.1007/s00382-009-0708-3, 2010.
Roberts, C. D., LeGrande, A. N., and Tripati, A. K.: Sensitivity of seawater oxygen isotopes to climatic and tectonic boundary conditions in an early Paleogene simulation with GISS ModelE-R, Paleoceanography, 26, PA4203, https://doi.org/10.1029/2010PA002025, 2011.
Rohling, E. J.: Oxygen isotope composition of seawater, in: The Encyclopedia of Quaternary Science, Elsevier, Amsterdam, 915–922, ISBN 978-0-444-53642-6, 2013.
Rohling, E. J., Yu, J., Heslop, D., Foster, G. L., Opdyke, B., and Roberts, A. P.: Sea level and deep-sea temperature reconstructions suggest quasi-stable states and critical transitions over the past 40 million years, Sci. Adv., 7, eabf5326, https://doi.org/10.1126/sciadv.abf5326, 2021.
Rosenheim, B. E., Swart, P. K., and Willenz, P.: Calibration of sclerosponge oxygen isotope records to temperature using high-resolution δ18O data, Geochim. Cosmochim. Ac., 73, 5308–5319, https://doi.org/10.1016/j.gca.2009.05.047, 2009.
Shackleton, N. J.: Attainment of isotopic equilibrium between ocean water and the benthonic foraminifera genus Unigerina: isotopic changes in the ocean during the last glacial, 219, Centre Natl. Rech. Sci. Coll. Inter., 203–209, https://epic.awi.de/id/eprint/32862/ (last access: 7 June 2023), 1974.
Sharp, Z.: Principles of Stable Isotope Geochemistry, 2nd Edn., University of New Mexico Open Textbooks, 416 pp., https://doi.org/10.25844/h9q1-0p82, 2017.
Singh, H. K. A., Donohoe, A., Bitz, C. M., Nusbaumer, J., and Noone, D. C.: Greater aerial moisture transport distances with warming amplify interbasin salinity contrasts, Geophys. Res. Lett., 43, 8677–8684, https://doi.org/10.1002/2016GL069796, 2016.
Spero, H. J., Bijma, J., Lea, D. W., and Bemis, B. E.: Effect of seawater carbonate concentration on foraminiferal carbon and oxygen isotopes, Nature, 390, 497–500, https://doi.org/10.1038/37333, 1997.
Storm-Suke, A., Dempson, J. B., Reist, J. D., and Power, M.: A field-derived oxygen isotope fractionation equation for Salvelinus species, Rapid Commun. Mass Spectrom., 21, 4109–4116, https://doi.org/10.1002/rcm.3320, 2007.
Thorrold, S. R., Campana, S. E., Jones, C. M., and Swart, P. K.: Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish, Geochim. Cosmochim. Ac., 61, 2909–2919, https://doi.org/10.1016/S0016-7037(97)00141-5, 1997.
Tierney, J. E., Zhu, J., King, J., Malevich, S. B., Hakim, G. J., and Poulsen, C. J.: Glacial cooling and climate sensitivity revisited, Nature, 584, 569–573, https://doi.org/10.1038/s41586-020-2617-x, 2020.
Tindall, J., Flecker, R., Valdes, P., Schmidt, D. N., Markwick, P., and Harris, J.: Modelling the oxygen isotope distribution of ancient seawater using a coupled ocean–atmosphere GCM: Implications for reconstructing early Eocene climate, Earth Planet. Sc. Lett., 292, 265–273, https://doi.org/10.1016/j.epsl.2009.12.049, 2010.
Tremaine, D. M., Froelich, P. N., and Wang, Y.: Speleothem calcite farmed in situ: Modern calibration of δ18O and δ13C paleoclimate proxies in a continuously-monitored natural cave system, Geochim. Cosmochim. Ac., 75, 4929–4950, https://doi.org/10.1016/j.gca.2011.06.005, 2011.
Tyrrell, T. and Zeebe, R. E.: History of carbonate ion concentration over the last 100 million years, Geochim. Cosmochim. Ac., 68, 3521–3530, https://doi.org/10.1016/j.gca.2004.02.018, 2004.
Veizer, J. and Prokoph, A.: Temperatures and oxygen isotopic composition of Phanerozoic oceans, Earth-Sci. Rev., 146, 92–104, https://doi.org/10.1016/j.earscirev.2015.03.008, 2015.
Westerhold, T., Marwan, N., Drury, A. J., Liebrand, D., Agnini, C., Anagnostou, E., Barnet, J. S. K., Bohaty, S. M., Vleeschouwer, D. D., Florindo, F., Frederichs, T., Hodell, D. A., Holbourn, A. E., Kroon, D., Lauretano, V., Littler, K., Lourens, L. J., Lyle, M., Pälike, H., Röhl, U., Tian, J., Wilkens, R. H., Wilson, P. A., and Zachos, J. C.: An astronomically dated record of Earth's climate and its predictability over the last 66 million years, Science, 369, 1383–1387, https://doi.org/10.1126/science.aba6853, 2020.
White, R. M. P., Dennis, P. F., and Atkinson, T. C.: Experimental calibration and field investigation of the oxygen isotopic fractionation between biogenic aragonite and water, Rapid Commun. Mass Spectrom., 13, 1242–1247, https://doi.org/10.1002/(SICI)1097-0231(19990715)13:13<1242::AID-RCM627>3.0.CO;2-F, 1999.
Willmes, M., Lewis, L. S., Davis, B. E., Loiselle, L., James, H. F., Denny, C., Baxter, R., Conrad, J. L., Fangue, N. A., Hung, T.-C., Armstrong, R. A., Williams, I. S., Holden, P., and Hobbs, J. A.: Calibrating temperature reconstructions from fish otolith oxygen isotope analysis for California's critically endangered Delta Smelt, Rapid Commun. Mass Spectrom., 33, 1207–1220, https://doi.org/10.1002/rcm.8464, 2019.
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K.: Trends, Rhythms, and Aberrations in Global Climate 65 Ma to Present, Science, 292, 686–693, https://doi.org/10.1126/science.1059412, 2001.
Zachos, J. C., Stott, L. D., and Lohmann, K. C.: Evolution of Early Cenozoic marine temperatures, Paleoceanography, 9, 353–387, https://doi.org/10.1029/93PA03266, 1994.
Zeebe, R. E. and Tyrrell, T.: History of carbonate ion concentration over the last 100 million years II: Revised calculations and new data, Geochim. Cosmochim. Ac., 257, 373–392, https://doi.org/10.1016/j.gca.2019.02.041, 2019.
Zhou, J., Poulsen, C. J., Pollard, D., and White, T. S.: Simulation of modern and middle Cretaceous marine δ18O with an ocean-atmosphere general circulation model, Paleoceanography, 23, PA3223, https://doi.org/10.1029/2008PA001596, 2008.
Zhu, J., Poulsen, C. J., Otto-Bliesner, B. L., Liu, Z., Brady, E. C., and Noone, D. C.: Simulation of early Eocene water isotopes using an Earth system model and its implication for past climate reconstruction, Earth Planet. Sc. Lett., 537, 116164, https://doi.org/10.1016/j.epsl.2020.116164, 2020.
Ziveri, P., Thoms, S., Probert, I., Geisen, M., and Langer, G.: A universal carbonate ion effect on stable oxygen isotope ratios in unicellular planktonic calcifying organisms, Biogeosciences, 9, 1025–1032, https://doi.org/10.5194/bg-9-1025-2012, 2012.