the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Chironomid- and pollen-based quantitative climate reconstructions for the post-Holsteinian (MIS 11b) in Central Europe
Tomasz Polkowski
Agnieszka Gruszczyńska
Bartosz Kotrys
Artur Górecki
Anna Hrynowiecka
Marcin Żarski
Mirosław Błaszkiewicz
Jerzy Nitychoruk
Monika Czajkowska
Stefan Lauterbach
Michał Słowiński
Investigating climatic and environmental changes during past interglacials is crucial to improve our understanding of the mechanisms that govern changes related to current global warming. Among the numerous proxies that can be used to reconstruct past environmental and climatic conditions, pollen allows quantitative reconstructions of annual, warmest month and coldest month air temperatures as well as precipitation sums, and Chironomidae larvae are widely used to infer past summer air temperature. Chironomidae have mostly been used for reconstructing Holocene and Late Weichselian summer temperatures whilst there are only four sites in Europe with chironomid-based summer air temperature reconstructions for the Late Pleistocene and no such records for any Middle Pleistocene warm period as of the writing of this paper. In this study we present the first quantitative palaeoclimate reconstruction for the post-Holsteinian (Marine Isotope Stage – (MIS) 11b) in Central Europe based on both pollen and fossil chironomid remains preserved in palaeolake sediments recovered from Krępa, southeastern Poland. Besides being used for the palaeoclimatic reconstruction, pollen analysis provides the biostratigraphic framework and a broader perspective of climate development at the end of Holsteinian Interglacial. Fossil Chironomidae assemblages at Krępa consist mainly of oligotrophic and mesotrophic taxa (e.g. Corynocera ambigua, Chironomus anthracinus-type) while eutrophic taxa (e.g. Chironomus plumosus-type) are less abundant. The chironomid-based summer temperature reconstruction indicates July air temperatures between 15.3 and 20.1 °C during the early post-Holsteinian, while pollen-based temperature reconstructions (using MAT and WA-PLS methods) indicate temperature values from 15 to 19 °C. Pollen-derived mean temperature of the coldest month (MTCO) and mean annual precipitation sum vary from −13.2 to −9.6 °C and between 500 and 900 mm respectively. In any case, results from Krępa prove that conducting Chironomidae analysis is feasible for periods as early as the Middle Pleistocene, improving our understanding of the mechanisms that control present-day climatic and environmental changes.
- Article
(5651 KB) - Full-text XML
-
Supplement
(290 KB) - BibTeX
- EndNote
Earth's history is characterised by repeated climate fluctuations, which had not been influenced by humans until the Holocene (marine isotope stage (MIS) 1), the most recent interglacial period. This offers the opportunity to compare natural climatic changes in the past with current ones in order to assess anthropogenic impact on the present climate. With respect to human impact during the Holocene, the so-called “Anthropocene” is widely debated across various scientific disciplines though its exact timing, and the actual dimension of human influence on the environment are still debated (Brondizio et al., 2016).
Holocene environmental archives, such as lake, palaeolake and ocean sediments provide material for comprehensive palaeoecological analyses. The sensitivity of some groups of organisms in these archives to changing hydrological or climatic conditions allows reconstruction of past events that directly affected the abundance or structure of the communities (Battarbee, 2000). Species, which are characterised by narrow ecological preferences, such as air temperature, water chemistry or water depth, are used for certain palaeoenvironmental reconstructions (Juggins and Birks, 2012). Many ecological parameters can be reconstructed using different proxies. For example, foraminiferas can be used to reconstruct ocean pH (Foster and Rae, 2016; Roberts et al., 2018), pollen can provide information about vegetation changes (Ralska-Jasiewiczowa et al., 2004; Kupryjanowicz et al., 2018) and can be used to reconstruct past human activity (Chevalier et al., 2020) or past climate conditions (e.g. Rylova and Savachenko, 2005; Hrynowiecka and Winter, 2016). Head capsules of chironomids can serve as the basis for summer air temperature reconstructions (Eggermont and Heiri, 2012) and for assessing the trophic state or pH of freshwater ecosystems (Płóciennik, 2005).
In general, palaeoecological and palaeoclimatological reconstructions record human impact on the environment from the Iron Age (Dumayne-Peaty, 1998; Szal et al., 2014). However, these reconstructions neither provide unequivocal information about air temperature changes nor allow the relative contribution of natural and human drivers to be distinguished. To gain a deeper understanding of the present human impact on climate and environment, it is therefore essential to investigate natural climate variability and environmental changes during past warm periods prior to any anthropogenic impact. In this regard, a particularly suitable targets are interglacial periods, e.g. Holsteinian Interglacial (or Mazovian Interglacial in Poland), which is commonly estimated to have lasted from 423 to 395 ka BP, thus corresponding to MIS 11c (Lauer and Weiss, 2018; Lauer et al., 2020; Fernández Arias et al., 2023). Holsteinian Interglacial is considered the analogue of the Holocene in terms of astronomical parameters (eccentricity, precession, insolation), climatic conditions and greenhouse gases levels (Koutsodendris et al., 2010; Yin and Berger, 2012; Kleinen et al., 2016). To date, there are only a few chironomid-based reconstructions of climatic and ecological conditions for the Middle and Late Pleistocene in Europe available (Engels et al., 2008; Bolland et al., 2021; Ilyashuk et al., 2022; Lapellegerie et al., 2024; Rigterink et al., 2024), but none for the Holsteinian Interglacial and the time thereafter. Hence, knowledge about climatic conditions at this time is mainly derived from Southern European pollen data, e.g. from the Praclaux maar in southern France (Reille and de Beaulieu, 1995), Tenaghi Philippon in north-eastern Greece (Tzedakis et al., 2006; Ardenghi et al., 2019), Lake Ohrid on the North Macedonian-Albanian border (Kousis et al., 2018), Lake Fucino in central Italy (Vera-Polo et al., 2024) and marine cores from ODP site 976 in the Alboran Sea (Sassoon et al., 2023, 2025) or from the North Atlantic off the Iberian coast (Oliveira et al., 2016). In Central Europe, high-resolution MIS 11 pollen records are available from the Ossówka palaeolake in eastern Poland (Nitychoruk et al., 2005, 2018; Bińka et al., 2023) as well as from Nowiny Żukowskie in eastern Poland (Hrynowiecka and Winter, 2016) and Dethlingen in northern Germany (Koutsodendris et al., 2010). Also notable is Bilhausen in central Germany, which provided a pollen record for the so-called Bilshausen Interglacial, which might correspond to MIS 11 or MIS 13 (Kühl and Gobet, 2010). In Northern Europe, there are even fewer records covering MIS 11 e.g. the record from Hoxne in eastern England (Horne et al., 2023) where temperature reconstructions were performed using chironomids (e.g., Brooks, 2006), ostracods (Horne, 2007) and beetle remains (Atkinson et al., 1987).
The contemporary state of knowledge on MIS 11 has been reviewed by Candy et al. (2014). Climate conditions in Central Europe were in general temperate at that time (Nitychoruk et al., 2018), but vegetation reconstructions suggest warmer and more humid conditions compared to the Holocene climatic optimum (Hrynowiecka and Winter, 2016). Two major climatic oscillations have so far been documented during the Holsteinian Interglacial, the Older Holsteinian Oscillation (OHO) and the Younger Holsteinian Oscillation (YHO). The OHO occurred around 418 ka BP (Koutsodendris et al., 2010, 2012; Górecki, 2023) and is clearly connected to a rapid cooling as indicated by the disappearance of temperate vegetation (mostly Picea-Alnus forests) and the spread of pioneer tree taxa including Betula, Pinus and Larix (Koutsodendris et al., 2010, 2012; Candy et al., 2014; Hrynowiecka and Pidek, 2017; Górecki et al., 2022). Although the OHO has been described at multiple sites across northern Europe (Koutsodendris et al., 2012), it has so far been identified in few southern European sites (Kousis et al., 2018; Sassoon et al., 2023, 2025). In contrast to the OHO, the YHO occurred around 400 ka BP within the climatic optimum of the Holsteinian Interglacial (Carpinus-Abies phase) and was apparently not connected to a significant cooling (Górecki et al., 2022). Records from Germany and eastern Poland suggest a sudden regression of Carpinus from forest communities (Koutsodendris et al., 2010; Hrynowiecka et al., 2019; Górecki et al., 2022). Particularly in Poland a rapid spread of Abies with an admixture of Corylus is observed, with Taxus also found in southern sites (Górecki et al., 2022), suggesting that temperature was not limiting the growth of Carpinus.
The climate during Holsteinian Interglacial (MIS 11c) was characterised by relatively stable warm and moist conditions with global temperatures approximately 1.5–2 °C above the pre-industrial level (Masson-Delmotte et al., 2010). Raymo and Mitrovica (2012) and Muhs et al. (2012) suggest sea level was possibly 6–13 m higher than present in this period. This can be partially attributed to the melting of the Greenland Ice Sheet (Robinson et al., 2017), as pollen and palaeoDNA data suggest the existence of spruce forests in Greenland at this time (Willerslev et al., 2007; de Vernal and Hillaire-Marcel, 2008).
In Europe, warm and wet oceanic climate conditions extended far to the east as evidenced by the presence of Taxus and Abies pollen at sites in Lithuania (Kondratiene and Gudelis, 1983), Belarus (Mamakowa and Rylova, 2007), and western Ukraine (Łanczont et al., 2003; Benham et al., 2016), whilst modern distribution limits of these taxa are estimated further to the west (Benham et al., 2016). Evidence from several terrestrial records from Eurasia suggests that the MIS 11c climate was highly complex, with pronounced climate variability occurring on both centennial and millennial timescales (Koutsodendris et al., 2010; Prokopenko et al., 2010; Tzedakis, 2010; Oliveira et al., 2016; Tye et al., 2016; Górecki et al., 2022).
The pollen succession of the Holsteinian Interglacial in Poland is characterised by a dominance of Picea-Alnus at first, then Carpinus and Abies, as well as a significant proportion of Taxus. Thermophilic taxa also occur frequently, examples including: Pterocarya, Celtis, Juglans, Ilex, Carya, Parrotia, Buxus, Vitis, Brasenia, Trapa, and Azolla (Janczyk-Kopikowa, 1991). Temperature reconstructions based on the indicator species method suggest the warmest period was the Carpinus-Abies phase, with estimated temperatures of 0–3 °C in January and 21–26 °C in July. This, along with high precipitation created a suitable environment for the spread of rare warmth-adapted taxa (Krupiński, 1995; Hrynowiecka and Winter, 2016). However, palaeotemperature reconstructions from Dethlingen (Koutsodendris et al., 2012) suggest slightly lower temperatures in Western Europe for both January (−2.2 ± 3.1 °C) and July (17.8 ± 2.1 °C). Pollen-based temperature reconstruction from Lake Ohrid (SE Europe) indicates higher January (MTCO) maximum (4.4 °C) (Kousis et al., 2018). The warm phase of the Holsteinian Interglacial was also confirmed by oxygen isotope analyses on endogenic lake carbonates (Nitychoruk et al., 2005) and snail shells (Szymanek, 2018). These showed significant changes in climatic conditions throughout the Holsteinian Interglacial, during which, continental and maritime influences intertwined in Central Europe. Continental influences resulted in a shortened vegetation period with long winters, whilst the opposite occurred under maritime influence, i.e. the vegetation period was significantly longer, temperatures were milder and precipitation rates were higher, also reflected by the appearance of stenothermal plant species (Nitychoruk et al., 2005).
Holsteinian Interglacial was followed by gradual cooling period (MIS 11b) which resulted in annual temperature decline and forest contractions (Tzedakis et al., 2006; Kousis et al., 2018; Hrynowiecka et al., 2019; Sassoon et al., 2025). MIS 11b brought the AP percentages decrease in Central Europe (Hrynowiecka et al., 2019). Lake Ohrid pollen record reveals the domination of Pinus and plant open communities at the time, with Poaceae and Artemisia species included (Kousis et al., 2018). ODP Site 976 pollen-based climate reconstructions shows annual temperature drop to around 10 °C and summer temperature to 20 °C (Sassoon et al., 2025).
Aiming at improving the knowledge about climate variability at the demise of the Holsteinian Interglacial, we present the first quantitative climate reconstructions for the post-Holsteinian in Central Europe, based on chironomid and pollen analyses of the Krępa core (southeastern Poland). The aim of analysing this post-interglacial period is to investigate temperature and vegetation changes and to determine if climate at the time was considerably cooler than today. This choice was also dictated by Chironomidae head capsules' presence in post-Holsteinian section of the Krępa core (unlike the Holsteinian part). In addition, we discuss the potential of chironomid analysis for palaeoecological study of Quaternary sediments as well as the challenges for chironomid analysis arising from both the evolution and interchanging adaptations to species ecological preferences and the preservation of fossil remains.
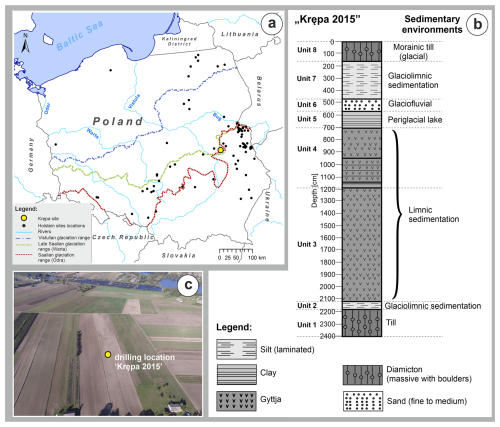
Figure 1(a) Location of selected sites with deposits from the Holsteinian Interglacial in Poland with the Krępa site indicated by the big yellow dot. Glaciation ranges are based on Żarski et al. (2024), Pochocka-Szwarc et al. (2024) and Marks (2023). (b) Lithological profile of the Krępa sediment succession and (c) location of the drilling site (picture M. Żarski).
2.1 Study area, coring and lithology
The Krępa palaeolake sediment succession (51°37′53.2′′ N, 22°18′38.1′′ E, 146 m a.s.l.) is located in SE Poland, near the city of Kock, approximately 120 km southeast of Warsaw (Fig. 1). It is under influence of humid continental climate (Dfb) in terms of the Köppen-Geiger climate classification (Peel et al., 2007). Average annual temperature for this region is ∼ 8.6 °C, with July mean temperature of ∼ 19 °C and January mean temperature of ∼ −1 °C, while average annual precipitation is ca. 600 mm (Ustrnul et al., 2021). Geomorphologically, it is situated in the central-eastern part of the North European Plain behind the maximum extent of the Saalian glaciation (Marks et al., 2018) and the sediment core analysed in this paper was obtained on a moraine plateau related to this ice sheet. Holsteinian Interglacial deposits in the area were first identified by Jesionkiewicz (1982) during cartographic work for the 1 : 50 000 Detailed Geological Map of Poland (DGMP; Sheet 676 – Kock) (Drozd and Trzepla, 2007). On the moraine plateau, the interglacial deposits are found under a thin cover of moraine deposits, whereas at the slopes of the nearby Wieprz River valley, they are exposed directly on the surface. This study's material was obtained from a sediment core that was drilled at Krępa in 2015, using a Geoprobe drilling device (Górecki, 2023).
The basal part of the 23.8 m-long sediment core that was recovered from the Krępa sediment succession in 2015 (Fig. 1) consisted of a 2 m-thick layer of light greyish brown sandy clays with a large number of rock fragments (unit 1), which is interpreted as till. As indicated by its stratigraphic position and its petrographic characteristics (Drozd and Trzepla, 2007), this till was likely accumulated during the Elsterian glaciation (Sanian 2 glaciation in Poland), which is considered to correspond to MIS 12. Directly above the till, a 0.6 m-thick layer of laminated sandy silts and sandy-clayey silts is present (unit 2). These sediments are interpreted as the result of glaciolimnic sedimentation in a relatively shallow water body between blocks of dead ice during the recession of the Elsterian ice-sheet. The glaciolimnic sediments of unit 2 gradually turn into a carbonate gyttja with small interlayers of carbonatic-minerogenic gyttja (unit 3), which was most likely deposited in the profundal zone of an already relatively deep lake. Between 1187 and 760 cm core depth, non-carbonatic organic-minerogenic gyttjas are found with mineral content generally increasing towards the top of the core (unit 4). The limnic sediments of unit 4 are interpreted to reflect the gradual shallowing of the lake due to continuous sediment infilling. At the same time, the systematic increase in mineral components in the sediments most likely reflects increased denudation and erosion in the catchment, possibly favoured by reduced vegetation cover in response to a climatic shift towards colder conditions. The gyttja sequence of unit 4 is overlain by a 1.9 m-thick layer of clays (unit 5), which probably represent accumulation in a periglacial lake. The following 1.1 m-thick layer of fine- to medium-grained sands (unit 6) as well as the overlying 3.1 m-thick layer of rhythmically laminated sandy silts (unit 7) are interpreted as proglacial sediments (units 6 and 7) of the transgressing Early Saalian (MIS 6) ice sheet. Above this, the profile is capped by a 1.5 m-thick layer of sandy morainic till with rock fragments (unit 8) related to Saalian glaciation.
Due to the inability to apply radiocarbon dating (e.g. 14C) and the challenges in developing an age–depth model, the direct dating of Holstein interglacial sediments is highly limited. In this context, palynology plays a key role, as pollen analysis enables biostratigraphic comparison between sites. Vegetation changes that occurred during the Holstein interglacial show a relatively uniform pattern across Central Europe from boreal phases to the development of thermophilus deciduous forests (Nitychoruk et al., 2005; Koutsodendris et al., 2010; Hrynowiecka and Winter, 2016) and cooling period (MIS 11b) thereafter (Hrynowiecka et al., 2019). Thanks to the repeatability of this vegetational succession, it is possible to correlate sediment profiles from different locations and assign them to a common stratigraphic framework. Thus, palynology becomes the primary tool for reconstructing and comparing environmental records from this period, despite the lack of precise absolute dating.
2.2 Chironomidae analysis
Initially, 79 sediment samples of 1 cm3, taken between 800 and 2160 cm depth at 5–40 cm intervals, were investigated for the presence of Chironomidae head capsules. However, only 30 of them (965–1155 cm depth) simultaneously contained more than 0–2 individuals. Chemical preparation followed Brooks et al. (2007). The precipitate was initially heated with KOH. The wet sediment was then passed through 212 µm (to remove larger sediment particles) and 100 µm mesh sieves and subsequent residues were treated in an ultrasonic bath for 3 s. The processed sediment was subsequently examined under a stereomicroscope (Zeiss Axio Lab A1) at 25× magnification. Chironomid head capsules from each sample were picked and mounted in Euparal. In case of damaged head capsules, individuals were counted as one if more than half of a body was preserved. Identification of chironomid head capsules followed Wiederholm (1983), Schmid (1993), Klink and Moller Pillot (2003), Brooks et al. (2007) and Andersen et al. (2013). Ecological preferences of identified taxa are based mainly on Brooks et al. (2007), Brundin (1949), Brodersen and Lindegaard (1999a) and Saether (1979).
Preliminary tests of sample preparation avoided the use of chemicals and included soaking the samples in water for a long time instead to reduce mechanical stress exerted to the head capsules during sample sieving as much as possible. Nevertheless, intact head capsules could not be extracted from some sediment samples even when using this gentle way of sample preparation, likely because of the already highly compacted sediment. As small numbers of head capsules may hinder palaeoecological and palaeoclimatic reconstructions, it was therefore partly necessary to combine samples (see below) or to increase the volume of the analysed sediment material (some samples were even as large as 20 cm3).
Chironomidae subfossil larvae were obtained from a total of 30 samples from the gyttja sediments (unit 4 on Fig. 1). Samples that contained fewer than 50 head capsules were merged except for a solitary sample at 1000 cm core depth. For 5 samples the required number of 50 head capsules was obtained and the remaining 24 samples were merged into seven clusters. After merging, sample clusters at 975, 1080, 1120 and 1125 cm core depth still did not reach 50 head capsules, but nonetheless, these samples and the one from 1000 cm core depth were included in the reconstruction as preliminary results seemed credible in terms of obtained temperature values.
2.3 Chironomid-based mean July air temperature reconstruction
In order to reconstruct mean July air temperatures (Tjul-Ch) from the Krępa chironomid assemblage, the Swiss-Norwegian-Polish (SNP) training set (Kotrys et al., 2020) was used as this covers a higher temperature span than other available European training sets (e.g. the Finnish, Russian, Swiss-Norwegian training sets) (Kotrys et al., 2020). The SNP training set includes 357 lakes, 134 taxa, covers a temperature range between 3.5 and 20.1 °C. Weighted averaging-partial least squares regression (WA-PLS) was used for performing the reconstruction. The Root Mean Square Error of Prediction (RMSEP) for this combined training set is 1.39 °C, and the R2 is 0.91 (Kotrys et al., 2020). Detrended Correspondence (MinDC) was also calculated. The temperature reconstruction was carried out using the C2 (v. 1.6) software (Juggins, 2007).
The lowest number of head capsules used for the Tjul-Ch reconstruction was 5 individuals at 1070 cm core depth whereas the highest number was 78 at 985 cm core depth. After merging, the total number of samples used for the Tjul-Ch reconstruction was 13.
2.4 Pollen analysis
The Krępa sediment core obtained in 2015 was sampled for palynological analyses at 5 cm intervals between 770 and 2180 cm depth, totaling 281 samples. A volume of 1 cm3 was collected from organic sediments (peat, gyttja), while minerogenic sediments (clays, silts, sands) were sampled with a volume of 3 cm3 due to the anticipated low pollen grain concentration. Samples were further processed following the standard methodology outlined by Erdtman (1960) with modifications such as the use of HF (Berglund and Ralska-Jasiewiczowa, 1986). Prior to laboratory processing, a Lycopodium tablet (Lund University, batch number 100 320 201, 20 408 ± 543 spores per tablet) was added to each sample to determine the absolute sporomorph concentration (Stockmarr, 1971). Pollen grains were counted using a ZEISS Axio Imager A2 light microscope. Palynomorphs were identified using pollen keys and atlases (Beug, 1961; Stuchlik, 2001, 2002, 2009; Lenarczyk, 2014), as well as online resources (PalDat, 2000; NPP Database, Shumilovskikh et al., 2022). For most samples, counts were conducted up to a sum of 500 pollen grains from arboreal (AP) and non-arboreal (NAP) plants. However, samples from glacial sediments with low palynomorph concentration were counted up to a sum of 300 pollen grains only. Percentages were calculated based on the sum of pollen grains from trees and shrubs (AP), as well as herbaceous plants, and dwarf shrubs (NAP). The results of the palynological analysis are depicted in a simplified pollen diagram (Fig. 3) that was plotted using R Studio with the package riojaPlot (Juggins, 2022). Local Pollen Assemblage Zones (LPAZ) were established using the CONISS cluster analysis function within riojaPlot and were visually adjusted if necessary.
2.5 Pollen-based climate reconstructions
Climate variables reconstructed using pollen data include mean annual air temperature (TANN), mean annual precipitation (PANN), mean temperature of the warmest month (MTWA), and mean temperature of the coldest month (MTCO). Two reconstruction approaches were applied: the Modern Analog Technique (MAT; Overpeck et al., 1985; Guiot, 1990) and Weighted Averaging Partial Least Squares regression (WA-PLS; ter Braak et al., 1993; ter Braak and Juggins, 1993). In the MAT approach, the best number of analogues (k) was chosen by comparing model performance (RMSE and R2) across k values from 1 to 10. This analysis indicated that using k=7 nearest analogues minimised prediction error, and thus 7 analogues were used in the final MAT reconstructions. For WA-PLS model selection, including the determination of the optimal number of components, was based on predictive accuracy assessed through leave-one-out (LOO) cross-validation and supported by randomization tests, following the methodology outlined by Chevalier et al. (2020). Based on these criteria, a four component WA-PLS was adopted. For each reconstruction model, the coefficient of determination (R2) and root mean square error (RMSE) were calculated to evaluate model performance. To express the uncertainty in the fossil climate reconstructions, we calculated standard errors of prediction (SEP) and depicted them as error bars in the figures. In the WA-PLS approach, sample-specific SEP were obtained via a bootstrapping implemented in the rioja package (Juggins, 2022). For the MAT model we used the cross-validated RMSE as a uniform error estimate for the fossil MAT reconstructions. Modern pollen data used in the reconstructions were sourced from the Northern Hemisphere database compiled by Herzschuh et al. (2023a, b). To enhance spatial relevance, the modern dataset was geographically filtered to include only samples within a 3000 km radius of the fossil site. This geographic filtering yielded a regional calibration set of 4955 modern pollen samples, out of the original global dataset. From the fossil pollen dataset, only taxa present in at least 50 % of the samples and reaching at least 1 % pollen value at least once were included. Additionally we ensured taxonomic consistency between the modern and fossil pollen data by harmonizing taxa names and then removing taxa with zero abundance in the filtered modern set. After this filtering, 10 pollen taxa remained in common between the modern calibration set and the fossil record (primarily major arboreal and herb taxa such as Larix, Betula, Pinus, Salix, Picea, J uniperus, Artemisia, Asteraceae, Poaceae, and Amaranthaceae). Using only these common taxa helps avoid noise from spurious taxa and improves model robustness. All data processing and modeling were carried out in R (RStudio), making use of the analogue and rioja packages for calibration and reconstruction.The pollen-based reconstructions were restricted to the interval of the succession where chironomid remains were also present and were performed on 44 samples.
3.1 Ecological reconstruction based on Chironomidae assemblages from the Krępa site
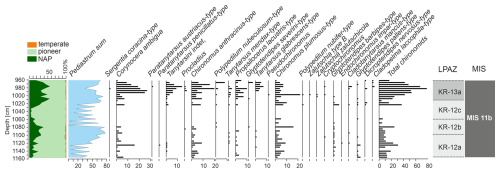
In general, the chironomid assemblages preserved in the Krępa sediments are dominated by two species Corynocera ambigua and Chironomus anthracinus-type.
Lower part of the sediment sequence (2180–1160 cm) is almost completely devoid of Chironomid remains, except few badly preserved Chironomus anthracinus, Chironomus plumosus and Glyptotendipes pallens head capsules at 2000, 1680, and 1205–1190 cm depths. Head capsules are recorded again at 1155–1122.5 cm depths – mostly Corynocera ambigua, Chironomus anthracinus, Chironomus plumosus and Glyptotendipes pallens (Fig. 2). 1072.5–1122.5 cm part predominantly contains cold-adapted species such as Corynocera ambigua and freeze-resistant species such as Glyptotendipes pallens-type and Glyptotendipes severini-type, which are often associated with algae and diatoms or mine leaves (Tarkowska-Kukuryk, 2014). 1022.5–1072.5 cm depth range is characterised by species highly resistant to difficult environmental conditions, such as Chironomus anthracinus-type, Corynocera ambigua and Glyptotendipes pallens-type From 1022.5 to 967.5 cm depth there are several species observed. This is the part most abundant in Chironomidae head capsules, with over 40 individuals per sample on average and maximum 78 head capsules at 985 cm sample. Species composition during this part is dominated by Corynocera ambigua, Chironomus anthracinus-type, Chironomus-plumosus-type and Propsilocerus lacustris-type. Additionally, some Tanytarsus glabrecens-type head capsules appear – this species was almost unseen in remaining sections. Between 967.5 and 877.5 cm depth, the number of chironomid head capsules started to decline above 965 cm depth, with only single unidentified Chironomidae head capsules at 955 and 950 cm. In subsequent section (877.5–765 cm) the number of Chironomidae is very low – only 2 Chironomus plumosus-type individuals were identified. Even Corynocera ambigua, abundant in previous sections, disappears.
3.2 July air temperature reconstruction based on Chironomidae assemblages from the Krępa site
Due to the low number of chironomid head capsules preserved in the Krępa sediments, a chironomid-based July temperature reconstruction was only possible for the uppermost part of the sediment core, encompassing the post-Holsteinian stadial that is most likely equivalent to MIS 11b. LPAZ KR-12a marks the onset of MIS 11b that directly follows the Holsteinian Interglacial. In this period, average July temperatures still ranged between 17 and 19 °C before rapidly dropping to about 16 °C and increasing again to 18–20 °C in LPAZ KR-12b (Fig. 4). July temperatures remained at this level throughout LPAZ KR-12c, before significantly dropping to 15–17 °C in the middle of LPAZ KR-13a. Only at the end of LPAZ KR-13a, which is equivalent to the transition to the following interstadial that most likely corresponds to MIS 11a, July temperatures markedly increased again to about 20 °C.
Table 1Temperature reconstruction from Chironomidae preserved in the Krępa sediments with reconstructed mean July air temperature (Tjul-Ch), error of the estimated Tjul-Ch, minimum dissimilarity between the chironomid assemblage in the Krępa sediments training set samples (MinDC), principal component analysis values (PCA) and corresponding LPAZ.
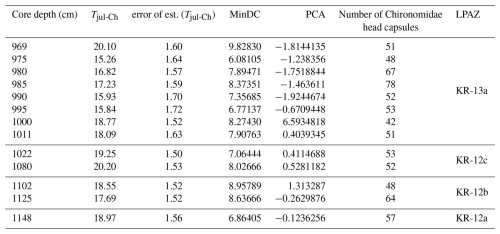
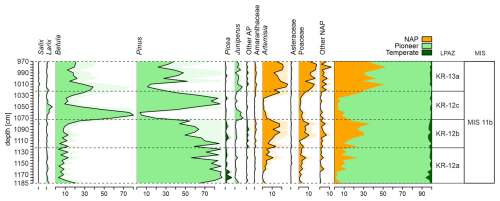
Figure 3Simplified percentage pollen diagram from the Krępa 2015 sediment core on depth scale (cm) with zonation of the diagram.
According to the SNP training set-based reconstruction, 10 samples with good modern analogues remain below the 5 % percentile threshold (minDC), while 3 samples with average modern analogues have values above the 5 % percentile threshold (6.08105< minDC >9.82830). PCA values range between ∼ −1.92 and 6.59 (Table 1).
3.3 Vegetation changes during the Early Saalian Glaciation at Krępa site and comparison with chironomid assemblages changes
Initially, 14 Local Pollen Assemblages Zones (LPAZ) covering the end of MIS 12 and MIS 11 period were extracted. Post-holsteinian (MIS 11b) covers LPAZ from 12a to 13a.
LPAZ KR-12a (1122.5–1187.5 cm) – At the beginning of the zone, the development of Pinus forests with an admixture of Picea (up to 6 %) is observed. Low NAP percentages suggest a very dense vegetation. However, percentages of Pinus and other tree species gradually decrease, and open herbaceous communities appear. The end of the zone is associated with a decrease in the percentage of Pinus pollen. Low number of Chironomidae head capsules (approximately 15 per sample). Dominance of Chironomus anthracinus-type (25 %) and Corynocera ambigua (16 %).
LPAZ KR-12b (1072.5–1122.5 cm) – A further decrease in Pinus pollen is observed. At the end of the zone, the landscape was likely already dominated by open communities (NAP up to 40 %) and sparse Pinus forests. Dominance of Corynocera ambigua (24 %) and high contents of Chironomus anthracinus-type. Disappearance of Glyptotendipes pallens-type and appearance of Glyptotendipes severini-type.
LPAZ KR-12c (1022.5–1072.5 cm) – Initially, dense Betula forests with Larix as an admixture dominated the landscape. Subsequently, a rapid development of Pinus forests is observed. The end of the zone is associated with a sudden drop in the percentage of Pinus pollen. The number of Chironomidae declines. Dominant species are Chironomus anthracinus-type (17 %), Corynocera ambigua and Glyptotendipes pallens-type (13 %).
LPAZ KR-13a (967.5–1022.5 cm) – Initially, there was a significant opening in the vegetation, and herbaceous plants and shrubs dominated the landscape. In the middle of this zone, there was a temporary return of very sparse Pinus and Betula forests, followed by another expansion of herbaceous vegetation. The end of the zone is associated with an increase in Betula pollen. Significant increase in the number of Chironomidae (on average 45 individuals per sample). Dominant species are Corynocera ambigua (approx. 29 %) and Chironomus anthracinus-type (18 %).
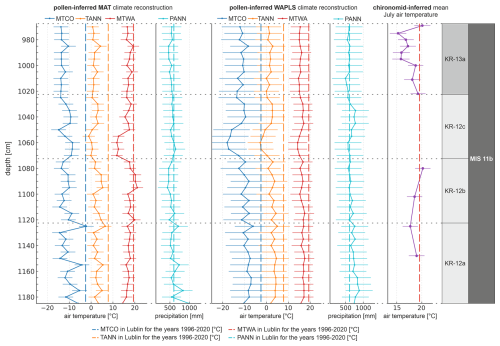
Figure 4Pollen-basedreconstructions of mean temperature of the warmest month (MTWA), mean annual temperature (TANN), mean temperature of the coldest month (MTCO), and annual precipitation sum (PANN) for the Krępa site using MAT and WA-PLS. Error bars indicate the standard error of prediction (SEP). The chironomid-based mean July air temperature reconstruction is given for comparison.
3.4 Pollen-based climate reconstructions from the Krępa site
Pollen-based climate reconstructions from the Krępa sediment core reveal distinct climate variability throughout MIS 11b, reflecting stadial–interstadial transitions (Fig. 4). The two pollen-based methods show broadly similar trends across all zones, with MAT generally producing higher summer temperature values than WA-PLS except in KR-12c. Where chironomid data are available, pollen-based MTWA reconstructions reproduce similar patterns, with differences falling within their respective uncertainty ranges. Among the two pollen-based models, MAT generally corresponds better to the chironomid WA-PLS reconstructions, showing overall closer alignment in reconstructed summer temperatures.
WA-PLS reconstructions were somewhat less robust, especially for precipitation, while the TANN and MTWA estimates still showed moderate predictive ability (Table S1 in the Supplement). Reconstructed MTWA from both pollen-based methods generally ranged between approximately 15 and 19 °C.
During LPAZ KR-12a, MAT- and WA-PLS-derived MTWA averaged approximately 16.8 °C, close to the chironomid-inferred mean of 18.3 °C. In LPAZ KR-12b, both pollen methods indicate further warming (∼ 18.7 °C MAT, ∼ 16.4 °C WA-PLS), consistent with the chironomid estimate of 19.4 °C, reflecting peak interstadial conditions. In LPAZ KR-12c, MTWA values dropped to ∼ 15.1 °C (MAT) and ∼ 15.7 °C (WA-PLS), indicating cooling during this interval. A moderate rebound is evident in LPAZ KR-13a, with MTWA increasing again to ∼ 17.3 °C (MAT) and ∼ 15.3 °C (WA-PLS), while the mean chironomid MTWA is 17.5 °C.
TANN values generally followed the summer temperature trends, beginning with relatively warm conditions in LPAZ KR-12a (∼ 3 °C). A slight increase was observed in LPAZ KR-12b (∼ 3.1 °C), followed by cooling in LPAZ KR-12c (∼ 1.2 °C). In LPAZ KR-13a, a modest recovery occurred with TANN rising to around 1.57 °C.
MTCO showed greater variability. Winters in LPAZ KR-12a and KR-12b were comparably cold, with MTCO values around −9.6 and −11.7 °C, respectively. LPAZ KR-12c showed slightly less severe winters (∼ −10.72 °C). A more pronounced cooling occurred in LPAZ KR-13a, where MTCO reached around −13.2 °C.
PANN reconstructions showed some uncertainty but generally ranged between 500 and 900 mm. LPAZ KR-12a was characterized by relatively high precipitation (∼ 640 mm), followed by moderately high values in LPAZ KR-12b (∼ 510 mm). A moderate increase occurred in LPAZ KR-12c (∼ 580 mm). In LPAZ KR-13a, PANN remained lower, typically around 520 mm, suggesting continued reduction in annual precipitation.
4.1 Chironomidae analysis as a method of palaeoclimate reconstruction
The analysis of subfossil Chironomidae is part of palaeoecological analysis conducted in geological, geomorphological, and archaeological research. Chironomidae, which are insects belonging to the suborder of Nematocera, are common, and inhabit various types of aquatic environments, from moist soil to lakes. Their development cycle can last from 20 days to several years as they can extend the duration of the larval stage depending on environmental conditions (Butler, 1982). Because of the excellent preservation of their larvae's head capsules in lake and peat bog sediments, the analysis of their subfossil remains offers the possibility to reconstruct environmental and climatic changes in the past. This includes quantitative reconstructions of the average July air temperature and the trophic state of the inhabited water body as well as the type and dynamics of the lake, the water pH, and microhabitats. Furthermore, training sets are also available to reconstruct the historic water level, salinity or oxygen content of the studied water body (Lotter et al., 1997).
4.1.1 Possible difficulties in temperature reconstruction based on Chironomidae analysis during past interglacials
The basic principle of palaeoecological reconstructions is uniformitarianism, implying that processes taking place on Earth in the past were the same as today (Krzeminski and Jarzembowski, 1999). This, for example, allows temperature to be reconstructed based on fossil Chironomidae assemblages by assuming that a given species has the same habitat requirements as thousands or hundreds of thousands years ago. The oldest recorded chironomid remains date back to the Late Triassic, i.e. ∼ 200 1 Ma BP (Krzeminski and Jarzembowski, 1999). Data from the MIS 11 Krępa sediments indicate a large difference in the number and state of preservation of chironomid remains compared to Holocene sites. Usually, at least 50 individuals per sample are required for robust reconstructions of the average July temperature, as smaller numbers of identified head capsules considerably increase the error range of the air temperature reconstruction. It is therefore commonly recommended to combine adjacent samples in case of low head capsule amounts (Heiri and Lotter, 2001). To enable selection of sites that could potentially yield chironomid-based palaeoenvironmental reconstructions, it is critical to analyse the factors that could limit the degree of preservation in chironomid remains, or cause a marked decrease/complete disappearance in the number of individuals.
Chironomidae inhabit all moist or aquatic habitats from moist wood to the ocean between the tropics and the Arctic. The high specialisation of individual species is thereby decisive for their common occurrence and their ability to survive under difficult environmental conditions. Among the features that allow specimens to succeed are: a short life cycle (in some cases only 8 d) (Reyes-Maldonado et al., 2021), osmoregulation, which enables survival in high-salinity waters (Kokkinn, 1986), or parthenogenesis, which implies a high efficiency of population reproduction, faster colonisation rate and high fertility (Lencioni, 2004; Nondula et al., 2004; Donato and Paggi, 2008; Orel and Semenchenko, 2019; Lackmann et al., 2020), as well as a short DNA chain (Gusev et al., 2010; Cornette et al., 2015). Some species are able to change food resources depending on the availability in their habitat (Tokeshi, 1995; Davis et al., 2003). Large lakes, such as the one that probably existed at Krępa (1) have a greater variety of habitats, thus being characterised by a larger biodiversity of Chironomidae (Allen et al., 1999; Heino, 2000; Tarr et al., 2005), and (2) are more resilient to extreme droughts and other extreme events. In contrast, small lakes with less diverse, isolated habitats exhibit reduced species diversity and dispersal (Roberts, 2003).
Despite the specialisation of chironomids, there are many conditions that limit the number of communities. One of the main factors limiting and determining the life processes of Chironomidae is temperature as each life stage is highly dependent on this factor. The development of eggs, larvae and pupae, nutrition and growth, the emergence of individuals and the ability to fly are all constrained by temperature maxima and minima, beyond which the given processes can no longer take place. Most groups can tolerate low sub-zero temperatures; the temperature below which the development of most species does not occur is −15 °C (Walker and Mathewes, 1989; Płóciennik, 2005). At Krępa, however, our July temperature reconstruction indicates temperatures well above that threshold, so even in case of severe winters, Chironomids should have been able to develop during the warmer periods of the year. Frost tolerance is highest in the Orthocladinae family and lowest in the Tanypodinae family (Danks, 1971). In the case of the Krępa sediments, species of both families were found (e.g. Propsilocerus lacustris-type and Procladius respectively) with Orthocladinae being more abundant than Tanypodinae (57 vs. 5 head capsules) with the highest number of head capsules being preserved during a period with relatively cool summers (15–17 °C).
Another important factor causing the decline of Chironomidae populations is the lack of oxygen in the water, although this cannot be directly captured by palaeoreconstructions. Instead, low-oxygen conditions are generally only indicated by an abundant occurrence of organic matter in the sediment. Such increases in organic matter commonly increase bacterial respiration and result in oxygen deficiency in the profundal of water bodies (Charlton, 1980; Matzinger et al., 2010; Müller et al., 2012). Another factor limiting the preservation of chironomid head capsules in sediments are mechanical factors that cause damage to the head capsules. For example, Tanypodinae remains are, due to their large size, not very resistant to disintegration and the number of preserved capsules may therefore be smaller (Walker et al., 1984). This would be consistent with our finding of only 5 Tanypodinae individuals in the Krępa sediments across four different depths. The preservation of remains only from the 3rd and 4th larval stages is most likely related to the increased amount of chitin in these developmental stages, making remains of these stages more resistant to disintegration. The remains of Chironomidae may also not be preserved if accumulation rate is low and remains of species from shore habitats could be poorly preserved. However, studies confirm a positive relationship between biocenosis and thanatocoenosis (Iovino, 1975; Walker et al., 1984). The number of generations per year may also affect the abundance of Chironomidae, i.e. subfossils of multivoltine species can be overrepresented compared to bivoltine species, however, it is difficult to determine whether changes in species composition correspond with voltinism (Tokeshi, 1995).
The main factor influencing the preservation of Chironomidae remains is the content of CaCO3, especially in moderately and strongly acidified lakes. This factor is often more important than pH, depth or time since the deposition of remains (Bailey et al., 2005). The microenvironment and the presence of organic matter are of great importance for the preservation of remains (Briggs and Kear, 1993; Sageman and Hollander, 1999). The faster mineralisation occurs, the better the preservation of any remains (Briggs and Kear, 1993; Park, 1995). Further factors reducing the abundance of chironomids are extreme temperatures, low nutrient levels, acidic waters, high Se concentrations (Del Wayne et al., 2018; Mousavi, 2002) , the content of hydrogen sulphide during holomixis, as well as paludification of the lake (Takagi et al., 2005; Płóciennik et al., 2020).
The lack of oxygen in the sediment could have limited not only the number of Chironomidae but also the number of preserved head capsules in the sediment. In particular, chitin does not usually accumulate in anaerobic sediment, because it is more easily broken down by bacteria, effectively mineralising it into CH4 and CO2 (Wörner and Pester, 2019).
Chironomid species found in the Krępa sediments have a wide range of environmental conditions in which they occur. In particular, we observe dominance of species resilient to harsh conditions, such as the oxygen-deficiency-resistant Chironomus anthracinus-type, the eutrophic Chironomus plumosus-type (18.7 % and 22.2 % of the total number of head capsules, respectively), as well as the cold-adapted Corynocera ambigua (25.7 %) and the freeze-resistant Propsilocerus lacustris-type (7.5 %).
Corynocera ambigua is a species often described as cold-adapted oligotrophic (Fjellberg, 1972; Pinder and Reiss, 1983; Walker and Mathewes, 1988; Brooks et al., 2007; Luoto et al., 2008; van Asch et al., 2012), inhabiting shallow lakes in arctic and subarctic regions, though it is also found in eutrophic lakes (Halkiewicz, 2008; Kotrys et al., 2020) Adults of this species are not able to fly, and breed on the water surface when the temperature reaches approximately 7–8 °C (optimum 13.7 °C). Mothes (1968) concluded that Corynocera ambigua larvae develop in autumn and winter, but not during summer. The decline in their numbers may be due to growth of filamentous algae in summer. Larvae of Corynocera ambigua are eurythermic, while the pupae are cold-stenothermic (Brundin, 1949). They only reproduce at low temperatures and inhabit water bodies with a maximum depth of approximately 25 m. The abundance of Corynocera ambigua has been shown to be correlated with charophyte contents (Brodersen and Lindegaard, 1999b). Although this species does not feed on charophytes, their presence may increase the number of diatoms and stabilise the trophic status and water clarity (Forsberg, 1965; Blindow, 1992). Corynocera ambigua live in dendritic tubes, its main food source being diatoms/algal detritus. (Fjellberg, 1972; Boubee, 1983).
This species has been recorded during cold episodes or glacial periods, at sites in England (Bedford et al., 2004), Norway (Velle et al., 2005), Poland (Płóciennik et al., 2015), and the Baltic region (Hofmann and Winn, 2000). However, Corynocera ambigua, cannot be considered merely a cold species. Some authors believe that its occurrence depends on high oxygen content in the water (Brodersen and Lindegaard, 1999a) and for other authors, it is a pioneer species that appears first after glacial retreat, similarly to Chironomus anthracinus-type (e.g. Heiri and Millet, 2005; Ilyashuk et al., 2005, 2013, 2022; Gandouin et al., 2016). Luoto and Sarmaja-Korjonen (2011) suggest this is how the species adapts to existing climatic conditions. The locally observed decline in Corynocera ambigua numbers in the Krępa sediments could also be attributed to changes in lake productivity related to changes in the environment. For example, when the production of soil and trees increased, the number of this species has been found to decrease (Magny et al., 2006; Larocque-Tobler et al., 2009).
Chironomus anthracinus-type occurs in various lake zones and is capable of surviving approximately 2–4 months of oxygen deficiency in water (Hamburger et al., 1994). It is a species which easily occupies niches that are inaccessible to others. According to some authors, it is a eutrophic (Kansanen, 1985; Brodersen and Lindegaard, 1999b) or cold-adapted species (Rohrig et al., 2004; Brooks et al., 2007; Płóciennik et al., 2011) and prefers soft, more organic sediments (McGarrigle, 1980). Therefore, the appearance of Chironomus anthracinus-type and Glyptotendipes pallens-type in the Krępa sediment may indicate the onset of eutrophication. Both Chironomus anthracinus-type and Corynocera ambigua are found in stratified lakes (e.g., Saether, 1979; Heiri, 2004). As we observe in our record, both species are relatively resistant to unfavourable environmental conditions, so possess a wide range of conditions in which they can occur.
Chironomus plumosus-type, also quite abundant in the sediment sequence, occurs in a wide range of habitats and is particularly resistant to anoxia (Saether, 1979; Brooks et al., 2007). Moreover, along with Dicrotendipes nervosus-type, this species is an indicator of progressive eutrophication (Brodersen and Lindegaard, 1999b).
Both eutrophic and oligotrophic species, as well as warm- and cold-adapted species, occur in the Krępa sediments.
The origin of the sedimentary basin at Krępa is difficult to interpret. Most sites with deposits from the Holsteinian Interglacial in this region of Poland are associated with tunnel valleys that formed during the Elsterian glaciation (Żarski et al., 2005; Nitychoruk et al., 2006). However, these sites are usually located beyond the maximum extent of the Older Saalian glaciation (Drenthe Stage in Germany; Odra glaciation in Poland; MIS 6) and thus, are subtly visible in the present surface morphology. In the case of Krępa, these deposits have been covered by the Older Saalian glacial advance, resulting in the complete transformation of the post-Elsterian landscape. Based on the geological cross section presented in the DGMP sheet 676 – Kock (Drozd and Trzepla, 2007) and the distribution of interglacial deposits in the study area (Jesionkiewicz, 1982), it can be inferred that the depression hosting the Krępa palaeolake was a relatively extensive kettle hole, formed during the recession of the Elsterian ice sheet.
As there are obviously only very few habitats where no invertebrates occur, the absence of chironomid remains during most of the Holsteinian Interglacial could be best explained by sediment-related disintegration and/or anoxic conditions at the bottom of a relatively deep lake. Another reason for the lack of remains could be the mineralisation of chitin. This would be in agreement with the parallel observed lack of cellulose remains from plants as well as with the very low number of Tanypodinae head capsules. However, satisfyingly explaining the lack of chironomid remains in most of the interglacial lake deposits requires further research and an in-depth comparison of our results with other lake sediments that lack chitinous remains.
4.1.2 Chironomid-inferred temperature reconstruction from the Krępa site in relation to pollen-based climate reconstructions
A chironomid-based July temperature reconstruction was only possible for the part of the Krępa sediment core that corresponds to LPAZ KR-12 and early LPAZ KR-13, which most likely corresponds to MIS 11b. Chironomid-based July temperatures during the early part of this interval (LPAZ KR-12a and LPAZ KR-12b) were probably still relatively high and stable, ranging from 19 to 21 °C, but dropping rapidly in LPAZ KR-12c and LPAZ KR-13a to 15–17 °C. The following increase to ∼ 20 °C at the top of LPAZ KR-13a possibly reflects the transition into the post-Holsteinian interstadial that corresponds to MIS 11a. This data indicates the July temperature maximum during the post-Holsteinian is consistent with the temperature range of the SNP training set (3.5–20.0 °C) (Kotrys et al., 2020). Comparing MIS 11 to the Holocene, it is crucial to mention that insolation patterns for both periods differ – MIS 11 was characterised by two insolation maxima, whilst there was only one (though more distinct) during the Holocene (Rohling et al., 2010). In fact, summer temperature increase during MIS 11b might be explained by increasing insolation.
In general, most Chironomidae remains in the Krępa sediments are found during cool periods, but are absent during warm periods. In contrast, Chironomidae were most abundant in LPAZ KR-12, which roughly corresponds to MIS 11b, the first cold phase after the Holsteinian Interglacial (Imbrie et al., 1984; Fawcett et al., 2011). To date, studies using subfossil Chironomidae to reconstruct past climate conditions mainly focused on the Weichselian Late Glacial and the Holocene (Gandouin et al., 2016; Nazarova et al., 2018; Druzhinina et al., 2020). As a result, there are very few chironomid-based July temperature reconstructions for the Late and Middle Pleistocene older than 20 ka BP available (Gandouin et al., 2007; Samartin et al., 2016; Plikk et al., 2019; Ilyashuk et al., 2020; Bolland et al., 2021; Lapellegerie et al., 2024; Rigterink et al., 2024), and no studies for the MIS 11 complex. In general, chironomid records from other sites and time intervals are characterised by a higher abundance and species diversity of Chironomidae, whilst at Krępa, Chironomidae occur only during the early glacial period following the Holsteinian Interglacial. A similar phenomenon has so far only been observed in the Laptev Sea region (Arctic Siberia), where Chironomidae also appear only in the cold period after the Eemian Interglacial, when the site was surrounded by wet grass-sedge shrub tundra period (Andreev et al., 2004). Assemblages from this site consist mostly of unidentified Tanytarsini individuals, eutrophic Chironomus plumosus and semi-aquatic taxa such as Limnophyes/Paralimnophyes, Smittia and Paraphaenocladius. The three species from the latter group were not identified at Krępa as opposed to Chironomus plumosus and Tanytarsini. Contrary to the chironomid-based temperature reconstruction, the pollen-basedreconstructions using MAT and WA-PLS provide continuous temperature and precipitation records (Mauri et al., 2015; Chevalier et al., 2020). During LPAZ KR-12a, pollen reconstructions indicate relatively stable and moderate summer temperatures. Additionally, PANN remains relatively high during this phase, suggesting consistently moist conditions supporting dense forest coverage.
The significant NAP increase LPAZ KR-12b suggests substantial forest decline, although the pollen-based MTWA reconstructions indicate relatively warm summers. This combination of ecological and climatic signals strongly suggests that the decline in forest cover was primarily driven by colder winter temperatures rather than summer thermal conditions. The pollen-based MTWA reconstruction confirms peak interstadial warmth in terms of summer temperatures are comparable to current mean July temperatures in Eastern Poland (Mauri et al., 2015; Kotrys et al., 2020; Gedminienė et al., 2025). Furthermore, the pollen-based TANN reconstruction also highlights peak interstadial warmth during LPAZ KR-12b, indicating overall favourable climatic conditions during the growing season. The pronounced increase in open-ground vegetation (NAP dominance) and herbaceous taxa thus likely reflects an ecological response to severe winter conditions, that restricted the establishment and survival of forest taxa, particularly those sensitive to extreme winter frosts (Körner and Paulsen, 2004; Harrington and Gould, 2015).
LPAZ KR-12c begins with pioneer Betula-Larix forests, reflecting a significant climatic shift towards colder and possibly drier conditions. The appearance of Larix, a cold-tolerant, light-demanding taxon adapted to short growing seasons and low temperatures, reinforces the interpretation of subarctic or boreal-like climate conditions. Larix is typically associated with northern coniferous forests, and reaches its distributional limits in areas with low winter temperatures and moderate precipitation (San-Miguel-Ayanz et al., 2022). Gradually increasing pollen signals from Pinus indicate a modest rise in thermal conditions later within LPAZ KR-12c, but within a generally cool and moisture-limited climatic regime. The absence of chironomids during this interval corroborates the interpretation of sustained cooler and drier conditions. Chironomid assemblages are sensitive to environmental harshness, and under extremely cold or oligotrophic conditions, their production may be so low that remains are not preserved in sediment records (Eggermont and Heiri, 2012).
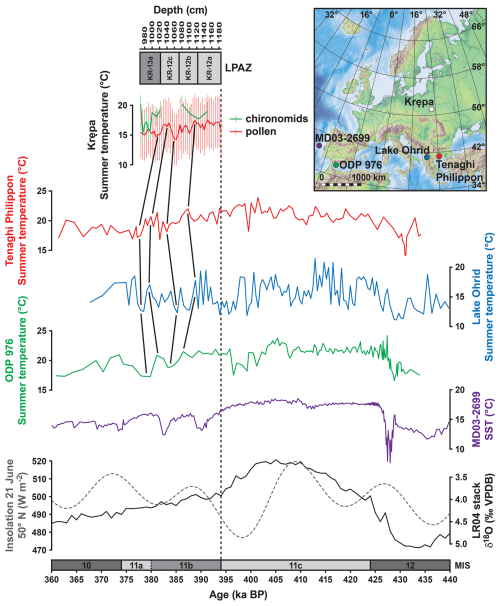
Figure 5Comparison of (top to bottom) the Marine Isotope Stage (MIS) 11b pollen- and chironomid-based summer temperature reconstructions from Krępa, a summer temperature reconstruction based on branched glycerol dialkyl glycerol tetraethers (brGDGTs) from Tenaghi Philippon, Greece (Ardenghi et al., 2019), a pollen-based summer temperature reconstruction from Lake Ohrid, Balkan Peninsula (Kousis et al., 2018; Kountsodendris et al., 2020), a pollen-based summer temperature reconstruction from ODP Site 976, Alboran Sea (Sassoon et al., 2025), and a biomarker-based (Uk'37) sea surface temperature (SST) reconstruction from marine core MD03-2699, Iberian margin (Rodrigues et al., 2011). The LR04 d18O stack (solid black line; Lisiecki and Raymo, 2005) and the 21 June insolation at 50° N (approximate latitude of Krępa; dashed grey line; Laskar et al., 2004) are provided as a palaeoclimatic context. The timing of the MIS boundaries 12/11c and 11a/10 is given according to Lisiecki and Raymo (2005); the timing of the MIS boundaries 11c/11b and 11b/11a is tentative. The insert map shows the locations of the individual proxy records.
The gradual cooling indicated by our chironomid-based reconstruction during LPAZ KR-13a is consistent with the presence of sparse Betula forests at the onset of this zone. Pollen-based reconstructions suggest that MTWA remained relatively mild (∼ 17.3 °C MAT, ∼ 15.3 °C WA-PLS), closely aligning with the chironomid-inferred mean Tjul-Ch value of ∼ 17.5 °C for this interval. Although the chironomid data exhibits a broader range (15–20 °C), this variability falls within typical reconstruction uncertainties and does not suggest a fundamentally different climatic signal. Meanwhile, declines in TANN and especially MTCO indicate cold-season severity remained the primary constraint on forest development (Nienstaedt, 1967; Körner and Paulsen, 2004; Harrington and Gould, 2015). In parallel, a reduction in PANN further supports increasing climatic stress, potentially limiting moisture availability and forest resilience during this transitional phase (Körner and Paulsen, 2004).
The broader relevance of the climatic conditions reconstructed from Krępa pollen data is given by the comparison with other MIS 11 palaeotemperature reconstructions from Southern Europe (Fig. 5) (Rodrigues et al., 2011; Oliveira et al., 2016; Kousis et al., 2018; Ardenghi et al., 2019; Sassoon et al., 2023, 2025). These Mediterranean records indicate generally warm conditions during MIS 11b, punctuated by recurrent cooling and drying events. For instance, the Lake Ohrid from SE Europe record shows a transition from temperate deciduous to cold mixed forests, with TANN dropping to ∼ 2 °C and mean temperature of the coldest month below −8 °C during the coldest events, despite precipitation often remaining near or above 800–900 mm (Kousis et al., 2018). Meanwhile, records from the SW Mediterranean reflect similar climate oscillations, with Sassoon et al. (2025) documenting synchronous declines in TANN and PANN centered ∼ 398 ka BP. Krępa record reflects relatively steady summer cooling alongside more marked declines in winter temperatures and moderately decreasing precipitation. Mediterranean vegetation is primarily water-limited, making it especially vulnerable to fluctuations in atmospheric moisture and reductions in winter rainfall (Giorgi, 2006; Lionello et al., 2006). Vegetation in Eastern Europe, however, is highly responsive to winter climate extremes. In particular, cold-season frost events, snow cover variability, and late-winter cold snaps affect plant performance, especially in temperate and continental zones (Kreyling, 2010; Camarero et al., 2022).
The lack of accurate absolute dating for terrestrial sediment sequences from the Holsteinian Interglacial makes it difficult to directly compare the results from Krępa to other MIS 11 sites. However, as there are a few quantitative temperature reconstructions based on pollen and biomarkers from other sites in Europe for the post-Holsteinian, a general comparison of temperature levels during this interval is feasible. For example, Tenaghi Philippon record indicates mild summer temperature drop to ∼ 16 °C at the coolest period of MIS 11b (Ardenghi et al., 2019). Climatic fluctuations at another Mediterranean region site – ODP 976 at Alboran Sea – were not abrupt, especially during first half of MIS 11b. Initially summer temperature stayed above 20 °C, only at further stage decreasing to ∼ 17 °C (Fig. 5) (Sassoon et al., 2025). Pollen analyses on marine sediments from the Iberian margin show similar climatic and ecological patterns for MIS 11b as observed at Krępa, namely repeated forest decline events. These were paralleled by reductions in sea surface temperature, although temperatures were still relatively high during most of MIS 11b – only about 1 °C below MIS 11c levels (Rodrigues et al., 2011; Oliveira et al., 2016). A similar pattern between still relatively high air temperature during early MIS 11b, and a temperature drop only during late MIS 11b is also seen in palynological data from Lake Ohrid in SE Europe (Kousis et al., 2018).
In line with our chironomid-based July temperature reconstruction from Krępa, these results show that the temperature decline at the demise of the Holsteinian Interglacial was not abrupt, and at least summer temperatures likely remained at a relatively high level for several thousand years. The general summer temperature variability that is seen in the Krępa record throughout the post-Holsteinian, i.e. the initial drop during the early MIS 11b, the following increase and the more pronounced decrease during late MIS 11b, as well as the marked increase at transition into MIS 11a, closely resembles vegetation and sea surface temperature variability at the Iberian margin, and may indicate a substantial impact of insolation variability (Rodrigues et al., 2011; Oliveira et al., 2016).
This study presents the first combined chironomid- and pollen-based palaeoclimatic reconstruction for the post-Holsteinian i.e. MIS 11b, offering a new perspective on climate variability in Eastern Europe during this period. The results highlight the complementarity and reliability of both proxies, as pollen-based MAT and WA-PLS reconstructions show strong internal consistency and correspond well with chironomid-inferred summer temperatures where data is available. The summer temperatures range from 15 to 19 °C and between 15 and 20 °C for the pollen- and chironomid-based reconstruction respectively. This indicates colder summers compared to present times for most of the post-Holsteinian period. The pollen-based MAT reconstructions exhibit particularly high predictive skill, especially for temperature variables. The analysed part of the Krępa sediment record reveals a progressive shift towards a more continental climate throughout MIS 11b. This is reflected by gradually cooling summers, increasingly severe winters, and a decline in annual precipitation.
To date, the vast majority of studies addressing terrestrial palaeoclimate variability during the Middle Pleistocene relies on pollen analysis. However, this does not imply a complete lack or low abundance of Chironomid-inferred reconstruction in sites other than Holocene. Moreover, they may prove to be a priceless source of knowledge on temperature, considering potential differences between pollen and Chironomid-inferred records. By comparing the results from different sites, it will be possible to identify the factor(s) that influenced the preservation of Chironomidae subfossil remains.
Ultimately, this study underscores the value of multi-proxy approaches in palaeoclimate reconstruction, particularly for pre-Holocene periods. Chironomids show significant potential as a summer temperature proxy in older sediments, as long as preservation conditions are favourable.
The pollen-based climatic reconstructions were conducted using established methods implemented in publicly available R packages. Specifically, the modern analogue technique (MAT) was applied using the R package analogue (Simpson and Oksanen, 2025), and the weighted-averaging partial least-squares (WAPLS) method was applied using the R package rioja (Juggins, 2024). The R code used in this study is not publicly available, as it only relies on standard functionality provided by these packages, which are fully documented in the corresponding CRAN repositories.
The dataset underlying this study is openly available at Zenodo (https://doi.org/10.5281/zenodo.17378964, Kotrys et al., 2025).
The supplement related to this article is available online at https://doi.org/10.5194/cp-21-1779-2025-supplement.
TP proposed the idea of the main text, and contributed to the figures. TP, AGr and AG wrote the original draft version of the manuscript. BK performed the chironomid-inferred summer temperature reconstruction. AG performed the pollen-inferred climate reconstructions. MŻ collected and described the core in the field. AG, AH, and MS analysed the pollen data. TP, AGr and MS analysed the chironomid data. TP, AGr, AG,, SL, MB and MS did the visualisations (graphs and maps). AG, AH, MŻ, MB, JN, MC, SL and MS reviewed the paper. All authors have made substantial contributions to the submission of this manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We would like to thank Harry Roberts (IGSO PAS) for proofreading and text corrections. The authors sincerely appreciate reviewers' and editor's valuable and constructive comments, which improved the quality of this manuscript.
This research has beed supported by the Narodowe Centrum Nauki (National Science Center) (grant. no. 2019/34/E/ST10/00275).
This paper was edited by Odile Peyron and reviewed by two anonymous referees.
Allen, A. P., Whittier, T. R., Kaufmann, P. R., Larsen, D. P., O'Connor, R. J., Hughes, R. M., Stemberger, R. S., Dixit, S. S., Brinkhurst, R. O., Herlihy, A. T., and Paulsen, S. G.: Concordance of taxonomic richness patterns across multiple assemblages in lakes of the northeastern United States, Can. J. Fish. Aquat. Sci., 56, 739–747, 1999.
Andersen, T., Sæther, O., Cranston, P., and Epler, J.: 9. The larvae of Orthocladiinae (Diptera: Chironomidae) of the Holarctic Region – Keys and diagnoses, in: The larvae of Chironomidae (Diptera) of the Holarctic region – Keys and diagnoses, edited by: Andersen, T., Cranston, P. S., and Epler J. H., Insect Syst. Evol. Suppl., 66, 189–386, 2013.
Andreev, A. A., Grosse, G., Schirrmeister, L., Kuzmina, S. A., Novenko, E. Yu., Bobrov, A. A., Tarasov, P. E., Ilyashuk, B. P., Kuznetsova, T. V., Krbetschek, M., Meyer, H., and Kunitsky, V. V.: Late Saalian and Eemian palaeoenvironmental history of the Bol'shoy Lyakhovsky Island (Laptev Sea region, Arctic Siberia), Boreas, 33, 319–348, https://doi.org/10.1111/j.1502-3885.2004.tb01244.x, 2004.
Ardenghi, N., Mulch, A., Koutsodendris, A., Pross, J., Kahmen, A., and Niedermeyer, E. M.: Temperature and moisture variability in the eastern Mediterranean region during Marine Isotope Stages 11–10 based on biomarker analysis of the Tenaghi Philippon peat deposit, Quat. Sci. Rev., 225, 105977, https://doi.org/10.1016/j.quascirev.2019.105977, 2019.
Atkinson, T. C., Briffa, K. R., and Coope, G. R.: Seasonal temperatures in Britain during the past 22,000 years, reconstructed using beetle remains, Nature, 325, 587–592, https://doi.org/10.1038/325587a0, 1987.
Bailey, J. V., Cohen, A. S., and Kring, D. A.: Lacustrine Fossil Preservation in Acidic Environments: Implications of Experimental and Field Studies for the Cretaceous–Paleogene Boundary Acid Rain Trauma, Palaios, 20, 376–389, https://doi.org/10.2110/palo.2003.p03-88, 2005.
Battarbee, R. W.: Palaeolimnological approaches to climate change, with special regard to the biological record, Quat. Sci. Rev., 19, 107–124, 2000.
Bedford, A., Jones, Richard. T., Lang, B., Brooks, S., and Marshall, J. D.: A Late-glacial chironomid record from Hawes Water, northwest England, J. Quat. Sci., 19, 281–290, https://doi.org/10.1002/jqs.836, 2004.
Benham, S., Durrant, T., Caudullo, G., and de Rigo, D.:TTaxus baccata in Europe: distribution, habitat, usage and threats, Eur. Atlas For. Tree Species, e015921, 2016.
Berglund, B. E. and Ralska-Jasiewiczowa, M.: Pollen analysis and pollen diagrams, Handb. Holocene Palaeoecol. Palaeohydrology, 455, 484–486, 1986.
Beug, H.-J.: Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete, G. Fischer, https://cir.nii.ac.jp/crid/19711493848636326 (last access: October 2025), 1961.
Bińka, K., Szymanek, M., and Nitychoruk, J.: Climate and vegetation changes recorded in the post Holsteinian lake deposits at Ossówka (eastern Poland), Acta Geol. Pol., 341–354, 2023.
Blindow, I.: Decline of charophytes during eutrophication: comparison with angiosperms, Freshw. Biol., 28, 9–14, https://doi.org/10.1111/j.1365-2427.1992.tb00557.x, 1992.
Bolland, A., Kern, O. A., Allstädt, F. J., Peteet, D., Koutsodendris, A., Pross, J., and Heiri, O.: Summer temperatures during the last glaciation (MIS 5c to MIS 3) inferred from a 50,000-year chironomid record from Füramoos, southern Germany, Quat. Sci. Rev., 264, 107008, https://doi.org/10.1016/j.quascirev.2021.107008, 2021.
Boubee, J. A. T.: Past and present benthic fauna of Lake Maratoto with special reference to the chironomidae, The University of Waikato, https://hdl.handle.net/10289/12748 (last access: October 2025), 1983.
Briggs, D. E. G. and Kear, A. J.: Fossilization of Soft Tissue in the Laboratory, Science, 259, 1439–1442, https://doi.org/10.1126/science.259.5100.1439, 1993.
Brodersen, K. P. and Lindegaard, C.: Classification, assessment and trophic reconstruction of Danish lakes using chironomids, Freshw. Biol., 42, 143–157, https://doi.org/10.1046/j.1365-2427.1999.00457.x, 1999a.
Brodersen, K. P. and Lindegaard, C.: Mass occurance and sporadic distribution of Corynocera ambigua Zetterstedt (Diptera, Chironomidae) in Danish lakes. Neo- and palaeolimnological records, J. Paleolimnol., 22, 41–52, https://doi.org/10.1023/A:1008032619776, 1999b.
Brondizio, E. S., O'Brien, K., Bai, X., Biermann, F., Steffen, W., Berkhout, F., Cudennec, C., Lemos, M. C., Wolfe, A., Palma-Oliveira, J., and Chen, C.-T. A.: Re-conceptualizing the Anthropocene: A call for collaboration, Glob. Environ. Change, 39, 318–327, https://doi.org/10.1016/j.gloenvcha.2016.02.006, 2016.
Brooks, S. J.: Fossil midges (Diptera: Chironomidae) as palaeoclimatic indicators for the Eurasian region, Quat. Sci. Rev., 25, 1894–1910, https://doi.org/10.1016/j.quascirev.2005.03.021, 2006.
Brooks, S. J., Langdon, P. G., and Heiri, O.: The identification and use of Palaearctic Chironomidae larvae in palaeoecology, Quat. Res. Assoc. Tech. Guide, i–vi, 1, 2007.
Brundin, L.: Chironomiden und andere bodentiere der südschwedischen urgebirgsseen : Ein beitrag zur kenntnis der bodenfaunistischen charakterzüge schwedischer oligotropher seen, Fiskeristyrelsen, https://urn.kb.se/resolve?urn=urn:nbn:se:havochvatten:diva-403 (last access: October 2025), 1949.
Butler, M. G.: A 7-year life cycle for two Chironomus species in arctic Alaskan tundra ponds (Diptera: Chironomidae), Can. J. Zool., 60, 58–70, https://doi.org/10.1139/z82-008, 1982.
Camarero, J. J., Sánchez-Salguero, R., Sangüesa-Barreda, G., Lechuga, V., Viñegla, B., Seco, J. I., Taïqui, L., Carreira, J. A., and Linares, J. C.: Reply to the letter to editor regarding Camarero et al. (2021): Overgrazing and pollarding threaten Atlas cedar conservation under forecasted aridification regardless stakeholders' nature, For. Ecol. Manag., 503, 119779, https://doi.org/10.1016/j.foreco.2021.119779, 2022.
Candy, I., Schreve, D. C., Sherriff, J., and Tye, G. J.: Marine Isotope Stage 11: Palaeoclimates, palaeoenvironments and its role as an analogue for the current interglacial, Earth-Sci. Rev., 128, 18–51, https://doi.org/10.1016/j.earscirev.2013.09.006, 2014.
Charlton, M. N.: Hypolimnion Oxygen Consumption in Lakes: Discussion of Productivity and Morphometry Effects, Can. J. Fish. Aquat. Sci., 37, 1531–1539, https://doi.org/10.1139/f80-198, 1980.
Chevalier, M., Davis, B. A. S., Heiri, O., Seppä, H., Chase, B. M., Gajewski, K., Lacourse, T., Telford, R. J., Finsinger, W., Guiot, J., Kühl, N., Maezumi, S. Y., Tipton, J. R., Carter, V. A., Brussel, T., Phelps, L. N., Dawson, A., Zanon, M., Vallé, F., Nolan, C., Mauri, A., de Vernal, A., Izumi, K., Holmström, L., Marsicek, J., Goring, S., Sommer, P. S., Chaput, M., and Kupriyanov, D.: Pollen-based climate reconstruction techniques for late Quaternary studies, Earth-Sci. Rev., 210, 103384, https://doi.org/10.1016/j.earscirev.2020.103384, 2020.
Cornette, R., Gusev, O., Nakahara, Y., Shimura, S., Kikawada, T., and Okuda, T.: Chironomid Midges (Diptera, Chironomidae) Show Extremely Small Genome Sizes, Zoolog. Sci., 32, 248–254, https://doi.org/10.2108/zs140166, 2015.
Danks, H. V.: Overwintering of some north temperate and arctic chironomidae: II. Chironomid biology, Can. Entomol., 103, 1875–1910, https://doi.org/10.4039/Ent1031875-12, 1971.
Davis, S., Golladay, S. W., Vellidis, G., and Pringle, C. M.: Macroinvertebrate Biomonitoring in Intermittent Coastal Plain Streams Impacted by Animal Agriculture, J. Environ. Qual., 32, 1036–1043, https://doi.org/10.2134/jeq2003.1036, 2003.
Del Wayne, R. N., Herrmann, S. J., Sublette, J. E., Melnykov, I. V., Helland, L. K., Romine, J. A., Carsella, J. S., Herrmann-Hoesing, L. M., Turner, J. A., and Heuvel, B. D. V.: Occurrence of Chironomid Species (Diptera: Chironomidae) in the High Se-78 Concentrations and High pH of Fountain Creek Watershed, Colorado, USA, West. North Am. Nat., 78, 39–64, https://doi.org/10.3398/064.078.0106, 2018.
de Vernal, A. and Hillaire-Marcel, C.: Natural Variability of Greenland Climate, Vegetation, and Ice Volume During the Past Million Years, Science, 320, 1622–1625, https://doi.org/10.1126/science.1153929, 2008.
Donato, M. and Paggi, A. C.: Polypedilum parthenogeneticum (Diptera: Chironomidae): a new parthenogenetic species from Eryngium L. (Apiaceae) phytotelmata, Aquat. Insects, 30, 51–60, https://doi.org/10.1080/01650420701829633, 2008.
Drozd, M. and Trzepla, M.: Objaśnienia do Szczegółowej mapy geologicznej Polski 1:50 000, Arkusz Kock (676), https://bazadata.pgi.gov.pl/data/smgp/arkusze_txt/smgp0676.pdf (last access: October 2025), 2007.
Druzhinina, O., Kublitskiy, Y., Stančikaitė, M., Nazarova, L., Syrykh, L., Gedminienė, L., Uogintas, D., Skipityte, R., Arslanov, K., Vaikutienė, G., Kulkova, M., and Subetto, D.: The Late Pleistocene–Early Holocene palaeoenvironmental evolution in the SE Baltic region: a new approach based on chironomid, geochemical and isotopic data from Kamyshovoye Lake, Russia, Boreas, 49, 544–561, https://doi.org/10.1111/bor.12438, 2020.
Dumayne-Peaty, L.: Human Impact on the Environment during the Iron Age and Romano-British Times: Palynological Evidence from Three Sites near the Antonine Wall, Great Britain, J. Archaeol. Sci., 25, 203–214, https://doi.org/10.1006/jasc.1997.0205, 1998.
Eggermont, H. and Heiri, O.: The chironomid-temperature relationship: expression in nature and palaeoenvironmental implications, Biol. Rev., 87, 430–456, https://doi.org/10.1111/j.1469-185X.2011.00206.x, 2012.
Engels, S., Bohncke, S. J. P., Bos, J. A. A., Brooks, S. J., Heiri, O., and Helmens, K. F.: Chironomid-based palaeotemperature estimates for northeast Finland during Oxygen Isotope Stage 3, J. Paleolimnol., 40, 49–61, https://doi.org/10.1007/s10933-007-9133-y, 2008.
Erdtman, G.: Pollen walls and angiosperm phylo-geny., Bot. Not., 113, 41–45, https://doi.org/10.5555/19601603471, 1960.
Fawcett, P. J., Werne, J. P., Anderson, R. S., Heikoop, J. M., Brown, E. T., Berke, M. A., Smith, S. J., Goff, F., Donohoo-Hurley, L., Cisneros-Dozal, L. M., Schouten, S., Sinninghe Damsté, J. S., Huang, Y., Toney, J., Fessenden, J., WoldeGabriel, G., Atudorei, V., Geissman, J. W., and Allen, C. D.: Extended megadroughts in the southwestern United States during Pleistocene interglacials, Nature, 470, 518–521, https://doi.org/10.1038/nature09839, 2011.
Fernández Arias, S., Förster, M. W., and Sirocko, F.: Rieden tephra layers in the Dottinger Maar lake sediments: Implications for the dating of the Holsteinian interglacial and Elsterian glacial, Glob. Planet. Change, 227, 104143, https://doi.org/10.1016/j.gloplacha.2023.104143, 2023.
Fjellberg, A.: Present and late Weichselian occurrence of Corynocera ambigua Zett.(Dipt., Chironomidae) in Norway, Entomol Tidsskr, 19, 59–61, http://www.entomologi.no/journals/nje/old/V19/NET_19_01_1972.pdf (last access: October 2025), 1972.
Forsberg, C.: Nutritional Studies of Chara in Axenic Cultures, Physiol. Plant., 18, 275–290, https://doi.org/10.1111/j.1399-3054.1965.tb06890.x, 1965.
Foster, G. L. and Rae, J. W. B.: Reconstructing Ocean pH with Boron Isotopes in Foraminifera, Annu. Rev. Earth Planet. Sci., 44, 207–237, https://doi.org/10.1146/annurev-earth-060115-012226, 2016.
Gandouin, E., Ponel, P., Andrieu-Ponel, V., Franquet, É., Beaulieu, J.-L. de, Reille, M., Guiter, F., Brulhet, J., Lallier-Vergès, É., Keravis, D., Grafenstein, U. von, and Veres, D.: Past environment and climate changes at the last Interglacial/Glacial transition (Les Échets, France) inferred from subfossil chironomids (Insecta), Comptes Rendus Géoscience, 339, 337–346, https://doi.org/10.1016/j.crte.2007.03.002, 2007.
Gandouin, E., Rioual, P., Pailles, C., Brooks, S. J., Ponel, P., Guiter, F., Djamali, M., Andrieu-Ponel, V., Birks, H. J. B., Leydet, M., Belkacem, D., Haas, J. N., Van der Putten, N., and de Beaulieu, J. L.: Environmental and climate reconstruction of the late-glacial-Holocene transition from a lake sediment sequence in Aubrac, French Massif Central: Chironomid and diatom evidence, Palaeogeogr. Palaeoclimatol. Palaeoecol., 461, 292–309, https://doi.org/10.1016/j.palaeo.2016.08.039, 2016.
Gedminienė, L., Spiridonov, A., Stančikaitė, M., Skuratovič, Ž., Vaikutienė, G., Daumantas, L., and Salonen, J. S.: Temporal and spatial climate changes in the mid-Baltic region in the Late Glacial and the Holocene: Pollen-based reconstructions, Catena, 252, 108851, https://doi.org/10.1016/j.catena.2025.108851, 2025.
Giorgi, F.: Climate change hot-spots, Geophys. Res. Lett., 33, https://doi.org/10.1029/2006GL025734, 2006.
Górecki, A.: Plant succession as an indicator of climatic oscillations during Masovian Interglacial (MIS 11c), PhD Thesis, https://ruj.uj.edu.pl/xmlui/bitstream/handle/item/319201/gorecki_abstract_2023.pdf?sequence=3&isAllowed=y (last access: October 2025), 2023.
Górecki, A., Żarski, M., Drzewicki, W., Pleśniak, Ł., Zalewska-Gałosz, J., and Hrynowiecka, A.: New climatic oscillations during MIS 11c in the record of the Skrzynka II site (Eastern Poland) based on palynological and isotope analysis, Quat. Int., 632, 4–20, https://doi.org/10.1016/j.quaint.2021.09.017, 2022.
Guiot, J.: Methodology of the last climatic cycle reconstruction in France from pollen data, Palaeogeogr. Palaeoclimatol. Palaeoecol., 80, 49–69, https://doi.org/10.1016/0031-0182(90)90033-4, 1990.
Gusev, O., Nakahara, Y., Vanyagina, V., Malutina, L., Cornette, R., Sakashita, T., Hamada, N., Kikawada, T., Kobayashi, Y., and Okuda, T.: Anhydrobiosis-Associated Nuclear DNA Damage and Repair in the Sleeping Chironomid: Linkage with Radioresistance, Plos One, 5, e14008, https://doi.org/10.1371/journal.pone.0014008, 2010.
Halkiewicz, A.: Corynocera ambigua (Insecta, Diptera) subfossils occurrence in recent sediments of four shallow Polesie lakes, in: Annales Universitatis Mariae Curie-Sklodowska, 31, https://doi.org/10.2478/v10067-008-0016-z, 2008.
Hamburger, K., Dall, P. C., and Lindegaard, C.: Energy metabolism of Chironomus anthracinus (Diptera: Chironomidae) from the profundal zone of Lake Esrom, Denmark, as a function of body size, temperature and oxygen concentration, Hydrobiologia, 294, 43–50, https://doi.org/10.1007/BF00017624, 1994.
Harrington, C. A. and Gould, P. J.: Tradeoffs between chilling and forcing in satisfying dormancy requirements for Pacific Northwest tree species, Front. Plant Sci., 6, 1–12, https://doi.org/10.3389/fpls.2015.00120, 2015.
Heino, J.: Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry, Hydrobiologia, 418, 229–242, https://doi.org/10.1023/A:1003969217686, 2000.
Heiri, O.: Within-lake variability of subfossil chironomid assemblages in shallow Norwegian lakes, J. Paleolimnol., 32, 67–84, https://doi.org/10.1023/B:JOPL.0000025289.30038.e9, 2004.
Heiri, O. and Lotter, A. F.: Effect of low count sums on quantitative environmental reconstructions: an example using subfossil chironomids, J. Paleolimnol., 26, 343–350, https://doi.org/10.1023/A:1017568913302, 2001.
Heiri, O. and Millet, L.: Reconstruction of Late Glacial summer temperatures from chironomid assemblages in Lac Lautrey (Jura, France), J. Quat. Sci., 20, 33–44, https://doi.org/10.1002/jqs.895, 2005.
Herzschuh, U., Böhmer, T., Li, C., Chevalier, M., Hébert, R., Dallmeyer, A., Cao, X., Bigelow, N. H., Nazarova, L., Novenko, E. Y., Park, J., Peyron, O., Rudaya, N. A., Schlütz, F., Shumilovskikh, L. S., Tarasov, P. E., Wang, Y., Wen, R., Xu, Q., and Zheng, Z.: LegacyClimate 1.0: a dataset of pollen-based climate reconstructions from 2594 Northern Hemisphere sites covering the last 30 kyr and beyond, Earth Syst. Sci. Data, 15, 2235–2258, https://doi.org/10.5194/essd-15-2235-2023, 2023a.
Herzschuh, U., Böhmer, T., Chevalier, M., Hébert, R., Dallmeyer, A., Li, C., Cao, X., Peyron, O., Nazarova, L., Novenko, E. Y., Park, J., Rudaya, N. A., Schlütz, F., Shumilovskikh, L. S., Tarasov, P. E., Wang, Y., Wen, R., Xu, Q., and Zheng, Z.: Regional pollen-based Holocene temperature and precipitation patterns depart from the Northern Hemisphere mean trends, Clim. Past, 19, 1481–1506, https://doi.org/10.5194/cp-19-1481-2023, 2023b.
Hofmann, W. and Winn, K.: The Littorina Transgression in the Western Baltic Sea as Indicated by Subfossil Chironomidae (Diptera) and Cladocera (Crustacea), Int. Rev. Hydrobiol., 85, 267–291, https://doi.org/10.1002/(SICI)1522-2632(200004)85:2/3<267::AID-IROH267>3.0.CO;2-Q, 2000.
Horne, D. J.: A Mutual Temperature Range method for Quaternary palaeoclimatic analysis using European nonmarine Ostracoda, Quat. Sci. Rev., 26, 1398–1415, https://doi.org/10.1016/j.quascirev.2007.03.006, 2007.
Horne, D. J., Ashton, N., Benardout, G., Brooks, S. J., Coope, G. R., Holmes, J. A., Lewis, S. G., Parfitt, S. A., White, T. S., Whitehouse, N. J., and Whittaker, J. E.: A terrestrial record of climate variation during MIS 11 through multiproxy palaeotemperature reconstructions from Hoxne, UK, Quat. Res., 111, 21–52, https://doi.org/10.1017/qua.2022.20, 2023.
Hrynowiecka, A. and Pidek, I. A.: Older and Younger Holsteinian climate oscillations in the palaeobotanical record of the Brus profile (SE Poland), Geol. Q., 61, 723–737, https://doi.org/10.7306/gq.1358, 2017.
Hrynowiecka, A. and Winter, H.: Palaeoclimatic changes in the Holsteinian Interglacial (Middle Pleistocene) on the basis of indicator-species method – Palynological and macrofossils remains from Nowiny Żukowskie site (SE Poland), Quat. Int., 409, 255–269, https://doi.org/10.1016/j.quaint.2015.08.036, 2016.
Hrynowiecka, A., Żarski, M., and Drzewicki, W.: The rank of climatic oscillations during MIS 11c (OHO and YHO) and post-interglacial cooling during MIS 11b and MIS 11a in eastern Poland, Geol. Q., 63, 375–394, https://doi.org/10.7306/gq.1470, 2019.
Ilyashuk, E. A., Ilyashuk, B. P., Hammarlund, D., and Larocque, I.: Holocene climatic and environmental changes inferred from midge records (Diptera: Chironomidae, Chaoboridae, Ceratopogonidae) at Lake Berkut, southern Kola Peninsula, Russia, The Holocene, 15, 897–914, https://doi.org/10.1191/0959683605hl865ra, 2005.
Ilyashuk, E. A., Ilyashuk, B. P., Kolka, V. V., and Hammarlund, D.: Holocene climate variability on the Kola Peninsula, Russian Subarctic, based on aquatic invertebrate records from lake sediments, Quat. Res., 79, 350–361, https://doi.org/10.1016/j.yqres.2013.03.005, 2013.
Ilyashuk, E. A., Ilyashuk, B. P., Heiri, O., and Spötl, C.: Summer temperatures and lake development during the MIS 5a interstadial: New data from the Unterangerberg palaeolake in the Eastern Alps, Austria, Palaeogeogr. Palaeoclimatol. Palaeoecol., 560, 110020, https://doi.org/10.1016/j.palaeo.2020.110020, 2020.
Ilyashuk, E. A., Ilyashuk, B. P., Heiri, O., and Spötl, C.: Summer temperatures and environmental dynamics during the Middle Würmian (MIS 3) in the Eastern Alps: Multi-proxy records from the Unterangerberg palaeolake, Austria, Quat. Sci. Adv., 6, 100050, https://doi.org/10.1016/j.qsa.2022.100050, 2022.
Imbrie, J., Hays, J. D., Martinson, D. G., McIntyre, A., Mix, A. C., Morley, J. J., Pisias, N. G., Prell, W. L., and Shackleton, N. J.: The orbital theory of Pleistocene climate: support from a revised chronology of the marine d18O record, 1, 269–305, https://epic.awi.de/id/eprint/41839/1/Imbrie-etal_1984.pdf (last access: October 2025), 1984.
Iovino, A. J.: Extant chironomid larval populations and the representativeness and nature of their remains in lake sediments., Indiana University, PhD Thesis, https://search.proquest.com/openview/0a29e11fa504f529ad04d21e50c89f98/1?pq-origsite=gscholar&cbl=18750&diss=y (last access: October 2025), 1975.
Janczyk-Kopikowa, Z.: The Ferdynand6w Interglacial in Poland, Geol. Q., 35, 71–80, 1991.
Jesionkiewicz, P.: Nowe stanowisko interglacjału mazowieckiego w Krępie koło Kocka, Geol. Q., 26, 423–430, 1982.
Juggins, S.: C2: Software for ecological and palaeoecological data analysis and visualisation (user guide version 1.5), Newctle. Tyne Newctle. Univ., 77, 680, https://www.academia.edu/download/84693763/C2.pdf (last access: October 2025), 2007.
Juggins, S.: riojaPlot: Stratigraphic diagrams in R, package version (0.1-20), https://cran.r-project.org/package=riojaPlot (last access: October 2025), 2022.
Juggins, S.: rioja: Analysis of Quaternary Science Data, R package version 1.0-7, CRAN [code], https://cran.r-project.org/package=rioja (last access: October 2025), 2024.
Juggins, S. and Birks, H. J. B.: Quantitative Environmental Reconstructions from Biological Data, in: Tracking Environmental Change Using Lake Sediments: Data Handling and Numerical Techniques, edited by: Birks, H. J. B., Lotter, A. F., Juggins, S., and Smol, J. P., Springer Netherlands, Dordrecht, 431–494, https://doi.org/10.1007/978-94-007-2745-8_14, 2012.
Kansanen, P. H.: Assessment of pollution history from recent sediments in Lake Vanajavesi, southern Finland. II. Changes in the Chironomidae, Chaoboridae and Ceratopogonidae (Diptera) fauna, Ann. Zool. Fenn., 22, 57–90, 1985.
Kleinen, T., Brovkin, V., and Munhoven, G.: Modelled interglacial carbon cycle dynamics during the Holocene, the Eemian and Marine Isotope Stage (MIS) 11, Clim. Past, 12, 2145–2160, https://doi.org/10.5194/cp-12-2145-2016, 2016.
Klink, A. G. and Moller Pillot, H.: Chironomidae larvae: key to the higher taxa and species of the lowlands of Northwestern Europe, https://www.cabidigitallibrary.org/doi/full/10.5555/20053034382 (last access: October 2025), 2003.
Kokkinn, M. J.: Osmoregulation, salinity tolerance and the site of ion excretion in the halobiont chironomid, Tanytarsus barbitarsis Freeman, Mar. Freshw. Res., 37, 243–250, https://doi.org/10.1071/mf9860243, 1986.
Kondratiene, O. and Gudelis, W.: Morskie osady plejstoceńskie na obszarze Pribałtyki, Przegląd Geol., 31, 497, 1983.
Körner, C. and Paulsen, J.: A world-wide study of high altitude treeline temperatures, J. Biogeogr., 31, 713–732, https://doi.org/10.1111/j.1365-2699.2003.01043.x, 2004.
Kotrys, B., Płóciennik, M., Sydor, P., and Brooks, S. J.: Expanding the Swiss-Norwegian chironomid training set with Polish data, Boreas, 49, 89–107, https://doi.org/10.1111/bor.12406, 2020.
Kotrys, B., Górecki, A., Hrynowiecka, A., Polkowski, T., Gruszczyńska, A., Lauterbach, S., Słowiński, M., Żarski, M., Błaszkiewicz, M., Czajkowska, M., andNitychoruk, J.: Chironomidae and pollen data for Krępa site, Zenodo [data set], https://doi.org/10.5281/zenodo.17378964, 2025.
Kousis, I., Koutsodendris, A., Peyron, O., Leicher, N., Francke, A., Wagner, B., Giaccio, B., Knipping, M., and Pross, J.: Centennial-scale vegetation dynamics and climate variability in SE Europe during Marine Isotope Stage 11 based on a pollen record from Lake Ohrid, Quat. Sci. Rev., 190, 20–38, https://doi.org/10.1016/j.quascirev.2018.04.014, 2018.
Koutsodendris, A.: Palynological data and pollen-based climate reconstructions from Lake Ohrid during MIS 12-11, PANGAEA, 2020. Mothes, G.: Einige ökologisch interessante Chironomiden aus dem Stechlinseegebiet, Ann. Zool. Fenn., 5, 92–96, 1968.
Koutsodendris, A., Müller, U. C., Pross, J., Brauer, A., Kotthoff, U., and Lotter, A. F.: Vegetation dynamics and climate variability during the Holsteinian interglacial based on a pollen record from Dethlingen (northern Germany), Quat. Sci. Rev., 29, 3298–3307, https://doi.org/10.1016/j.quascirev.2010.07.024, 2010.
Koutsodendris, A., Pross, J., Müller, U. C., Brauer, A., Fletcher, W. J., Kühl, N., Kirilova, E., Verhagen, F. T. M., Lücke, A., and Lotter, A. F.: A short-term climate oscillation during the Holsteinian interglacial (MIS 11c): An analogy to the 8.2 ka climatic event?, Glob. Planet. Change, 92–93, 224–235, https://doi.org/10.1016/j.gloplacha.2012.05.011, 2012.
Kreyling, J.: Winter climate change: A critical factor for temperate vegetation performance, Ecology, 91, 1939–1948, https://doi.org/10.1890/09-1160.1, 2010.
Krupiński, K. M.: Taxus in plant communities of the Mazovian interglacial age in Central Europe and its climatostratigraphical consequences, Bull. Pol. Acad. Sci. Earth Sci., 43, 29–41, http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-article-2cb6def3-0d71-4f06-801c-b8cf70351c17 (last access: October 2025), 1995.
Krzeminski, W. and Jarzembowski, E.: Aenne triassica sp.n., the oldest representative of the family Chironomidae [Insecta: Diptera], Pol. Pismo Entomol., 68, 445–449, http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-article-b1e269a8-12db-4907-aa7b-5759651a2eb2 (last access: October 2025), 1999.
Kühl, N. and Gobet, E.: Climatic evolution during the Middle Pleistocene warm period of Bilshausen, Germany, compared to the Holocene, Quat. Sci. Rev., 29, 3736–3749, https://doi.org/10.1016/j.quascirev.2010.08.006, 2010.
Kupryjanowicz, M., Nalepka, D., Pidek, I. A., Walanus, A., Balwierz, Z., Bińka, K., Fiłoc, M., Granoszewski, W., Kołaczek, P., Majecka, A., Malkiewicz, M., Nita, M., Noryśkiewicz, B., and Winter, H.: The east-west migration of trees during the Eemian Interglacial registered on isopollen maps of Poland, Quat. Int., 467, 178–191, https://doi.org/10.1016/j.quaint.2017.08.034, 2018.
Lackmann, A. R., McEwen, D. C., and Butler, M. G.: Evidence of parthenogenetic populations from the Paratanytarsus laccophilus species group (Diptera: Chironomidae) in the Alaskan Arctic, Chironomus J. Chironomidae Res., 48–58, https://doi.org/10.5324/cjcr.v0i33.3478, 2020.
Łanczont, M., Pidek, I. A., Bogucki, A., Wieliczkiewicz, F., and Wojtanowicz, J.: Kluczowy profil interglacjału mazowieckiego w Krukienicach na międzyrzeczu Sanu i Dniestru (Ukraina), Przegląd Geol., 51, 597–608, 2003.
Lapellegerie, P., Millet, L., Rius, D., Duprat-Oualid, F., Luoto, T., and Heiri, O.: Chironomid-inferred summer temperature during the Last Glacial Maximum in the Southern Black Forest, Central Europe, Quat. Sci. Rev., 345, 109016, https://doi.org/10.1016/j.quascirev.2024.109016, 2024.
Larocque-Tobler, I., Grosjean, M., Heiri, O., and Trachsel, M.: High-resolution chironomid-inferred temperature history since ad 1580 from varved Lake Silvaplana, Switzerland: comparison with local and regional reconstructions, The Holocene, 19, 1201–1212, https://doi.org/10.1177/0959683609348253, 2009.
Laskar, J., Robutel, P., Joutel, F., Gastineau, M., Correia, A. C. M., and Levrard, B.: A long-term numerical solution for the insolation quantities of the Earth, Astron. Astrophys., 428, 261–285, https://doi.org/10.1051/0004-6361:20041335, 2004.
Lauer, T. and Weiss, M.: Timing of the Saalian- and Elsterian glacial cycles and the implications for Middle – Pleistocene hominin presence in central Europe, Sci. Rep., 8, 5111, https://doi.org/10.1038/s41598-018-23541-w, 2018.
Lauer, T., Weiss, M., Bernhardt, W., Heinrich, S., Rappsilber, I., Stahlschmidt, M. C., von Suchodoletz, H., and Wansa, S.: The Middle Pleistocene fluvial sequence at Uichteritz, central Germany: Chronological framework, paleoenvironmental history and early human presence during MIS 11, Geomorphology, 354, 107016, https://doi.org/10.1016/j.geomorph.2019.107016, 2020.
Lenarczyk, J.: The Algal Genus “pediastrum Meyen” (chlorophyta) in Poland, W. Szafer Institute of Botany, Polish Academy of Sciences, https://www.botany.pl/images/Books/Lenarczyk_2014_The_algal_genus_Pediastrum_Meyen_Chlorophyta.pdf (last access: October 2025), 2014.
Lencioni, V.: Survival strategies of freshwater insects in cold environments, J. Limnol., 63, 45–55, https://agris.fao.org/search/en/providers/122415/records/6473690153aa8c89630d8c78 (last access: October 2025), 2004.
Lisiecki, L. E. and Raymo, M. E.: A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records, Paleoceanography, 20, https://doi.org/10.1029/2004PA001071, 2005.
Lionello, P., Malanotte-Rizzoli, P., Boscolo, R., Alpert, P., Artale, V., Li, L., Luterbacher, J., May, W., Trigo, R., Tsimplis, M., Ulbrich, U., and Xoplaki, E.: The Mediterranean climate: An overview of the main characteristics and issues, in: Developments in Earth and Environmental Sciences, vol. 4, edited by: Lionello, P., Malanotte-Rizzoli, P., and Boscolo, R., Elsevier, 1–26, https://doi.org/10.1016/S1571-9197(06)80003-0, 2006.
Lotter, A. F., Birks, H. J. B., Hofmann, W., and Marchetto, A.: Modern diatom, cladocera, chironomid, and chrysophyte cyst assemblages as quantitative indicators for the reconstruction of past environmental conditions in the Alps. I. Climate, J. Paleolimnol., 18, 395–420, https://doi.org/10.1023/A:1007982008956, 1997.
Luoto, T. P. and Sarmaja-Korjonen, K.: Midge-inferred Holocene effective moisture fluctuations in a subarctic lake, northern Lapland, Boreas, 40, 650–659, https://doi.org/10.1111/j.1502-3885.2011.00217.x, 2011.
Luoto, T. P., Nevalainen, L., and Sarmaja-Korjonen, K.: Multiproxy evidence for the `Little Ice Age' from Lake Hampträsk, Southern Finland, J. Paleolimnol., 40, 1097–1113, https://doi.org/10.1007/s10933-008-9216-4, 2008.
Magny, M., Aalbersberg, G., Bégeot, C., Benoit-Ruffaldi, P., Bossuet, G., Disnar, J.-R., Heiri, O., Laggoun-Defarge, F., Mazier, F., Millet, L., Peyron, O., Vannière, B., and Walter-Simonnet, A.-V.: Environmental and climatic changes in the Jura mountains (eastern France) during the Lateglacial–Holocene transition: a multi-proxy record from Lake Lautrey, Quat. Sci. Rev., 25, 414–445, https://doi.org/10.1016/j.quascirev.2005.02.005, 2006.
Mamakowa, K. and Rylova, T. B.: The interglacial from Korchevo in Belarus in the light of new palaeobotanical studies, Acta Palaeobot., 47, 425–453, http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-93eba296-a9fe-43c1-9599-40de8acc5417 (last access: October 2025), 2007.
Marks, L.: Quaternary stratigraphy of Poland–current status, Acta Geol. Pol., 73, 307–340, 2023.
Marks, L., Karabanov, A., Nitychoruk, J., Bahdasarau, M., Krzywicki, T., Majecka, A., Pochocka-Szwarc, K., Rychel, J., Woronko, B., Zbucki, Ł., Hradunova, A., Hrychanik, M., Mamchyk, S., Rylova, T., Nowacki, Ł., and Pielach, M.: Revised limit of the Saalian ice sheet in central Europe, Quat. Int., 478, 59–74, https://doi.org/10.1016/j.quaint.2016.07.043, 2018.
Masson-Delmotte, V., Stenni, B., Pol, K., Braconnot, P., Cattani, O., Falourd, S., Kageyama, M., Jouzel, J., Landais, A., Minster, B., Barnola, J. M., Chappellaz, J., Krinner, G., Johnsen, S., Röthlisberger, R., Hansen, J., Mikolajewicz, U., and Otto-Bliesner, B.: EPICA Dome C record of glacial and interglacial intensities, Quat. Sci. Rev., 29, 113–128, https://doi.org/10.1016/j.quascirev.2009.09.030, 2010.
Matzinger, A., Müller, B., Niederhauser, P., Schmid, M., and Wüest, A.: Hypolimnetic oxygen consumption by sediment-based reduced substances in former eutrophic lakes, Limnol. Oceanogr., 55, 2073–2084, https://doi.org/10.4319/lo.2010.55.5.2073, 2010.
Mauri, A., Davis, B. A. S., Collins, P. M., and Kaplan, J. O.: The climate of Europe during the Holocene: a gridded pollen-based reconstruction and its multi-proxy evaluation, Quat. Sci. Rev., 112, 109–127, https://doi.org/10.1016/j.quascirev.2015.01.013, 2015.
McGarrigle, M. L.: The Distribution of Chironomid Communities and Controlling Sediment Parameters in L. Derravaragh, Ireland, in: Chironomidae, edited by: Murray, D. A., Pergamon, 275–282, https://doi.org/10.1016/B978-0-08-025889-8.50043-X, 1980.
Mothes, G.: Einige ökologisch interessante Chironomiden aus dem Stechlinseegebiet, Ann. Zool. Fenn., 5, 92–96, 1968.
Mousavi, S. K.: Boreal chironomid communities and their relations to environmental factors: the impact of lake depth, size and acidity, Boreal Chironomid Communities Their Relat. Environ. Factors Impact Lake Depth Size Acidity, 7, 63–75, 2002.
Muhs, D. R., Pandolfi, J. M., Simmons, K. R., and Schumann, R. R.: Sea-level history of past interglacial periods from uranium-series dating of corals, Curaçao, Leeward Antilles islands, Quat. Res., 78, 157–169, https://doi.org/10.1016/j.yqres.2012.05.008, 2012.
Müller, B., Bryant, L. D., Matzinger, A., and Wüest, A.: Hypolimnetic Oxygen Depletion in Eutrophic Lakes, Environ. Sci. Technol., 46, 9964–9971, https://doi.org/10.1021/es301422r, 2012.
Nazarova, L. B., Subetto, D. A., Syrykh, L. S., Grekov, I. M., and Leontev, P. A.: Reconstructions of Paleoecological and Paleoclimatic Conditions of the Late Pleistocene and Holocene according to the Results of Chironomid Analysis of Sediments from Medvedevskoe Lake (Karelian Isthmus), Dokl. Earth Sci., 480, 710–714, https://doi.org/10.1134/S1028334X18060144, 2018.
Nienstaedt, H.: Chilling requirements in seven Picea species, Silvae Genet., 16, 65–68, 1967.
Nitychoruk, J., Bińka, K., Hoefs, J., Ruppert, H., and Schneider, J.: Climate reconstruction for the Holsteinian Interglacial in eastern Poland and its comparison with isotopic data from Marine Isotope Stage 11, Quat. Sci. Rev., 24, 631–644, https://doi.org/10.1016/j.quascirev.2004.07.023, 2005.
Nitychoruk, J., Bińka, K., Ruppert, H., and Schneider, J.: Holsteinian Interglacial=Marine Isotope Stage 11?, Quat. Sci. Rev., 25, 2678–2681, https://doi.org/10.1016/j.quascirev.2006.07.004, 2006.
Nitychoruk, J., Bińka, K., Sienkiewicz, E., Szymanek, M., Chodyka, M., Makos, M., Ruppert, H., and Tudryn, A.: A multiproxy record of the Younger Holsteinian Oscillation (YHO) in the Ossówka profile, eastern Poland, Boreas, 47, 855–868, https://doi.org/10.1111/bor.12308, 2018.
Nondula, N., Marshall, D. J., Baxter, R., Sinclair, B. J., and Chown, S. L.: Life history and osmoregulatory ability of Telmatogeton amphibius (Diptera, Chironomidae) at Marion Island, Polar Biol., 27, 629–635, https://doi.org/10.1007/s00300-004-0619-z, 2004.
Oliveira, D., Desprat, S., Rodrigues, T., Naughton, F., Hodell, D., Trigo, R., Rufino, M., Lopes, C., Abrantes, F., and Goni, M. F. S.: The complexity of millennial-scale variability in southwestern Europe during MIS 11, Quat. Res., 86, 373–387, https://doi.org/10.1016/j.yqres.2016.09.002, 2016.
Orel, O. and Semenchenko, A.: Morphological description and DNA barcodes of adult males of Tanytarsus heliomesonyctios Langton, 1999 (Diptera, Chironomidae) in northeast of Russia, Zootaxa, 4686, https://doi.org/10.11646/zootaxa.4686.1.6, 2019.
Overpeck, J. T., Iii, T. W., and Prentice, I. C.: Quantitative Interpretation of Fossil Pollen Spectra: Dissimilarity Coefficients and the Method of Modern Analogs, Quat. Res., 23, 87–108, https://doi.org/10.1016/0033-5894(85)90074-2, 1985.
PalDat: Palynological database, https://www.paldat.org (last access: October 2025), 2000.
Park, L. E.: Geochemical and Paleoenvironmental Analysis of Lacustrine Arthropod-Bearing Concretions of the Barstow Formation, Southern California, Palaios, 10, 44–57, https://doi.org/10.2307/3515006, 1995.
Peel, M. C., Finlayson, B. L., and McMahon, T. A.: Updated world map of the Köppen-Geiger climate classification, Hydrol. Earth Syst. Sci., 11, 1633–1644, https://doi.org/10.5194/hess-11-1633-2007, 2007.
Pinder, L. C. V. and Reiss, F.: The larvae of Chironominae (Diptera: Chironomidae) of the Holarctic region-Keys and diagnoses, Larvae, https://cir.nii.ac.jp/crid/1570854175201464448 (last access: October 2025), 1983.
Plikk, A., Engels, S., Luoto, T. P., Nazarova, L., Salonen, J. S., and Helmens, K. F.: Chironomid-based temperature reconstruction for the Eemian Interglacial (MIS 5e) at Sokli, northeast Finland, J. Paleolimnol., 61, 355–371, https://doi.org/10.1007/s10933-018-00064-y, 2019.
Płóciennik, M.: Zastosowanie subfosylnych szczątków ochotkowatych (Diptera: Chironomidae) w badaniach nad paleoklimatem i rekonstrukcją zmian w środowisku, Kosmos, 54, 401–406, 2005.
Płóciennik, M., Self, A., Birks, H. J. B., and Brooks, S. J.: Chironomidae (Insecta: Diptera) succession in Żabieniec bog and its palaeo-lake (central Poland) through the Late Weichselian and Holocene, Palaeogeogr. Palaeoclimatol. Palaeoecol., 307, 150–167, https://doi.org/10.1016/j.palaeo.2011.05.010, 2011.
Płóciennik, M., Kruk, A., Michczyńska, D. J., and Birks, J. B.: Kohonen Artificial Neural Networks and the IndVal Index as Supplementary Tools for the Quantitative Analysis of Palaeoecological Data, Geochronometria, 42, https://doi.org/10.1515/geochr-2015-0021, 2015.
Płóciennik, M., Pawłowski, D., Vilizzi, L., and Antczak-Orlewska, O.: From oxbow to mire: Chironomidae and Cladocera as habitat palaeoindicators, Hydrobiologia, 847, 3257–3275, https://doi.org/10.1007/s10750-020-04327-6, 2020.
Pochocka-Szwarc, K., Żarski, M., Hrynowiecka, A., Górecki, A., Pidek, I. A., Szymanek, M., Stachowicz-Rybka, R., Stachowicz, K., and Skoczylas–Śniaz, S.: Mazovian Interglacial sites in the Sosnowica Depression and the Parczew-Kodeń Heights (Western Polesie, SE Poland), and their stratigraphic, palaeogeographic and palaeoenvironmental significance, Geol. Q., 68, https://doi.org/10.7306/gq.1746, 2024.
Posit team: RStudio: Integrated Development Environment for R, Posit Software, PBC, Boston, MA, USA, http://www.posit.co/ (last access: October 2025), 2025.
Prokopenko, A. A., Bezrukova, E. V., Khursevich, G. K., Solotchina, E. P., Kuzmin, M. I., and Tarasov, P. E.: Climate in continental interior Asia during the longest interglacial of the past 500 000 years: the new MIS 11 records from Lake Baikal, SE Siberia, Clim. Past, 6, 31–48, https://doi.org/10.5194/cp-6-31-2010, 2010.
Ralska-Jasiewiczowa, M., Nalepka, D., and Goslar, T.: Some problems of forest transformation at the transition to the oligocratic/ Homo sapiens phase of the Holocene interglacial in northern lowlands of central Europe, Veg. Hist. Archaeobotany, 13, 71–71, https://doi.org/10.1007/s00334-003-0027-2, 2004.
Raymo, M. E. and Mitrovica, J. X.: Collapse of polar ice sheets during the stage 11 interglacial, Nature, 483, 453–456, https://doi.org/10.1038/nature10891, 2012.
Reille, M. and de Beaulieu, J.-L.: Long Pleistocene Pollen Records from the Praclaux Crater, South-Central France, Quat. Res., 44, 205–215, https://doi.org/10.1006/qres.1995.1065, 1995.
Reyes-Maldonado, R., Marie, B., and Ramírez, A.: Rearing methods and life cycle characteristics of Chironomus sp. Florida (Chironomidae: Diptera): A rapid-developing species for laboratory studies, Plos One, 16, e0247382, https://doi.org/10.1371/journal.pone.0247382, 2021.
Rigterink, S., Krahn, K. J., Kotrys, B., Urban, B., Heiri, O., Turner, F., Pannes, A., and Schwalb, A.: Summer temperatures from the Middle Pleistocene site Schöningen 13 II, northern Germany, determined from subfossil chironomid assemblages, Boreas, bor.12658, https://doi.org/10.1111/bor.12658, 2024.
Roberts, J., Kaczmarek, K., Langer, G., Skinner, L. C., Bijma, J., Bradbury, H., Turchyn, A. V., Lamy, F., and Misra, S.: Lithium isotopic composition of benthic foraminifera: A new proxy for paleo-pH reconstruction, Geochim. Cosmochim. Acta, 236, 336–350, https://doi.org/10.1016/j.gca.2018.02.038, 2018.
Roberts, M.: Investigation into the population dynamics and key life history characteristics of non-biting midge (Diptera: Chironomidae) which can be utilised to improve nuisance control at Lake Joondalup, Western Australia, Theses Honours, 2003.
Robinson, A., Alvarez-Solas, J., Calov, R., Ganopolski, A., and Montoya, M.: MIS-11 duration key to disappearance of the Greenland ice sheet, Nat. Commun., 8, 16008, https://doi.org/10.1038/ncomms16008, 2017.
Rodrigues, T., Voelker, A. H. L., Grimalt, J. O., Abrantes, F., and Naughton, F.: Iberian Margin sea surface temperature during MIS 15 to 9 (580–300 ka): Glacial suborbital variability versus interglacial stability, Paleoceanography, 26, https://doi.org/10.1029/2010PA001927, 2011.
Rohling, E. J., Braun, K., Grant, K., Kucera, M., Roberts, A. P., Siddall, M., and Trommer, G.: Comparison between Holocene and Marine Isotope Stage-11 sea-level histories, Earth Planet. Sci. Lett., 291, 97–105, https://doi.org/10.1016/j.epsl.2009.12.054, 2010.
Rohrig, R., Beug, H., Trettin, R., and Morgenstern, P.: Subfossil chironomid assemblages as paleoenvironmental indicators in lake faulersee (Germany), Stud. Quat., 117–127, 2004.
Rylova, T. and Savachenko, I.: Reconstruction of palaeotemperatures of pleistocene interglacial intervals of Belarus from palynological evidences, Pol. Geol. Inst. Spec. Pap., 16, 83–93, http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BUS6-0021-0064 (last access: October 2025), 2005.
Saether, O. A.: Chironomid communities as water quality indicators, Ecography, 2, 65–74, https://doi.org/10.1111/j.1600-0587.1979.tb00683.x, 1979.
Sageman, B. B. and Hollander, D. J.: Cross correlation of paleoecological and geochemical proxies: A holistic approach to the study of past global change, Spec. Pap. Geol. Soc. Am., 332, 365–384, https://doi.org/10.1130/0-8137-2332-9.365, 1999.
Samartin, S., Heiri, O., Kaltenrieder, P., Kühl, N., and Tinner, W.: Reconstruction of full glacial environments and summer temperatures from Lago della Costa, a refugial site in Northern Italy, Quat. Sci. Rev., 143, 107–119, https://doi.org/10.1016/j.quascirev.2016.04.005, 2016.
San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., and Mauri, A.: European atlas of forest tree species, Publication Office of the European Union, Luxembourg, https://doi.org/10.2788/4251, 2016.
Sassoon, D., Lebreton, V., Combourieu-Nebout, N., Peyron, O., and Moncel, M.-H.: Palaeoenvironmental changes in the southwestern Mediterranean (ODP site 976, Alboran sea) during the MIS 12/11 transition and the MIS 11 interglacial and implications for hominin populations, Quat. Sci. Rev., 304, 108010, https://doi.org/10.1016/j.quascirev.2023.108010, 2023.
Sassoon, D., Combourieu-Nebout, N., Peyron, O., Bertini, A., Toti, F., Lebreton, V., and Moncel, M.-H.: Pollen-based climatic reconstructions for the interglacial analogues of MIS 1 (MIS 19, 11, and 5) in the southwestern Mediterranean: insights from ODP Site 976, Clim. Past, 21, 489–515, https://doi.org/10.5194/cp-21-489-2025, 2025.
Schmid, P. E.: Random patch dynamics of larval Chironomidae (Diptera) in the bed sediments of a gravel stream, Freshw. Biol., 30, 239–255, https://doi.org/10.1111/j.1365-2427.1993.tb00806.x, 1993.
Shumilovskikh, L. S., Shumilovskikh, E. S., Schlütz, F., and van Geel, B.: NPP-ID: Non-Pollen Palynomorph Image Database as a research and educational platform, Veg. Hist. Archaeobotany, 31, 323–328, https://doi.org/10.1007/s00334-021-00849-8, 2022.
Simpson, G. L. and Oksanen, J.: analogue: Analogue and weighted averaging methods for palaeoecology, CRAN [code], https://doi.org/10.32614/CRAN.package.analogue, 2025.
Stockmarr, J.: 1971: Tablets with spores used in absolute pollen analysis, Pollen et Spores 13, 615–621, 1971.
Stuchlik, L.: Atlas of pollen and spores of the Polish Neogene, W. Szafer Institute of Botany, Polish Acadamy of Sciences, https://szukaj.bu.umk.pl/discovery/fulldisplay/alma991010006199706926/48OMNIS_UMKWT:UMK (last access: October 2025), 2001.
Stuchlik, L.: Atlas of Pollen and Spores of the Polish Neogene: Gymnosperms, W. Szafer Institute of Botany, Polish Acadamy of Sciences, https://biblioteka.botany.pl/cgi-bin/koha/opac-detail.pl?biblionumber=2338 (last access: October 2025), 2002.
Stuchlik, L.: Atlas of pollen and spores of the Polish Neogene. 3. Angiosperms (1), W. Szafer Inst. of Botany, Polish Academy of Sciences, https://biblioteka.botany.pl/bib/5239 (last access: October 2025), 2009.
Szal, M., Kupryjanowicz, M., Wyczółkowski, M., and Tylmann, W.: The Iron Age in the Mrągowo Lake District, Masuria, NE Poland: the Salęt settlement microregion as an example of long-lasting human impact on vegetation, Veg. Hist. Archaeobotany, 23, 419–437, https://doi.org/10.1007/s00334-014-0465-z, 2014.
Szymanek, M.: Elemental geochemistry of freshwater snail shells: palaeolimnology of a Holsteinian (MIS 11) deposit from eastern Poland, Boreas, 47, 643–655, https://doi.org/10.1111/bor.12283, 2018.
Takagi, S., Kikuchi, E., Doi, H., and Shikano, S.: Swimming Behaviour of Chironomus acerbiphilus Larvae in Lake Katanuma, Hydrobiologia, 548, 153–165, https://doi.org/10.1007/s10750-005-5196-9, 2005.
Tarkowska-Kukuryk, M.: Spatial distribution of epiphytic chironomid larvae in a shallow macrophyte-dominated lake: effect of macrophyte species and food resources, Limnology, 15, 141–153, https://doi.org/10.1007/s10201-014-0425-4, 2014.
Tarr, T. L., Baber, M. J., and Babbitt, K. J.: Macroinvertebrate community structure across a wetland hydroperiod gradient in southern New Hampshire, USA, Wetl. Ecol. Manag., 13, 321–334, https://doi.org/10.1007/s11273-004-7525-6, 2005.
ter Braak, C. J. F. and Juggins, S.: Weighted averaging partial least squares regression (WA-PLS): an improved method for reconstructing environmental variables from species assemblages, Hydrobiologia, 269, 485–502, https://doi.org/10.1007/BF00028046, 1993.
ter Braak, C. J. F., H., van D., Juggins, S., and Birks, H. J. B.: Weighted averaging partial least squares regression (WA-PLS): definition and comparison with other methods for species–environment calibration, in: Multivariate Environmental Statistics, edited by: Patil, G. P. and Rao, C. R., Elsevier, Amsterdam, the Netherlands, 525–556, https://edepot.wur.nl/249353 (last access: October 2025), 1993.
Tokeshi, M.: Life cycles and population dynamics, in: The Chironomidae: Biology and ecology of non-biting midges, edited by: Armitage, P. D., Cranston, P. S., and Pinder, L. C. V., Springer Netherlands, Dordrecht, 225–268, https://doi.org/10.1007/978-94-011-0715-0_10, 1995.
Tye, G. J., Sherriff, J., Candy, I., Coxon, P., Palmer, A., McClymont, E. L., and Schreve, D. C.: The δ18O stratigraphy of the Hoxnian lacustrine sequence at Marks Tey, Essex, UK: implications for the climatic structure of MIS 11 in Britain, J. Quat. Sci., 31, 75–92, https://doi.org/10.1002/jqs.2840, 2016.
Tzedakis, P. C.: The MIS 11 – MIS 1 analogy, southern European vegetation, atmospheric methane and the ”early anthropogenic hypothesis”, Clim. Past, 6, 131–144, https://doi.org/10.5194/cp-6-131-2010, 2010.
Tzedakis, P. C., Hooghiemstra, H., and Pälike, H.: The last 1.35 million years at Tenaghi Philippon: revised chronostratigraphy and long-term vegetation trends, Quat. Sci. Rev., 25, 3416–3430, https://doi.org/10.1016/j.quascirev.2006.09.002, 2006.
Ustrnul, Z., Wypych, A., Marosz, M., Biernacik, D., Czekierda, D., Chodubska, A., Wasielewska, K., Kusek, K., and Kopaczka, D.: Climate of Poland 2021, IMGW-PIB, https://www.imgw.pl/sites/default/files/2022-06/imgw-pib-klimat-polski-2021-eng-final.pdf (last access: October 2025), 2021.
van Asch, N., Kloos, M. E., Heiri, O., de Klerk, P., and Hoek, W. Z.: The younger dryas cooling in northeast Germany: summer temperature and environmental changes in the Friedländer Große Wiese region, J. Quat. Sci., 27, 531–543, https://doi.org/10.1002/jqs.2547, 2012.
Velle, G., Brooks, S. J., Birks, H. J. B., and Willassen, E.: Chironomids as a tool for inferring Holocene climate: an assessment based on six sites in southern Scandinavia, Quat. Sci. Rev., 24, 1429–1462, https://doi.org/10.1016/j.quascirev.2004.10.010, 2005.
Vera-Polo, P., Sadori, L., Jiménez-Moreno, G., Masi, A., Giaccio, B., Zanchetta, G., Tzedakis, P. C., and Wagner, B.: Climate, vegetation, and environmental change during the MIS 12-MIS 11 glacial-interglacial transition inferred from a high-resolution pollen record from the Fucino Basin of central Italy, Palaeogeogr. Palaeoclimatol. Palaeoecol., 655, 112486, https://doi.org/10.1016/j.palaeo.2024.112486, 2024.
Walker, I. R. and Mathewes, R. W.: Late-quaternary fossil chironomidae (diptera) from hippa lake, queen charlotte islands, british columbia, with special reference to corynocera zett., Can. Entomol., 120, 739–751, https://doi.org/10.4039/Ent120739-8, 1988.
Walker, I. R. and Mathewes, R. W.: Chironomidae (Diptera) remains in surficial lake sediments from the Canadian Cordillera: analysis of the fauna across an altitudinal gradient, J. Paleolimnol., 2, 61–80, https://doi.org/10.1007/BF00156985, 1989.
Walker, I. R., Fernando, C. H., and Paterson, C. G.: The chironomid fauna of four shallow, humic lakes and their representation by subfossil assemblages in the surficial sediments, Hydrobiologia, 112, 61–67, https://doi.org/10.1007/BF00007667, 1984.
Wiederholm, T.: Chironomidae of the holarctic region. Keys and diagnoses. Part 1: larva, Ent. Scand. Suppl., 19, 1–457, 1983.
Willerslev, E., Cappellini, E., Boomsma, W., Nielsen, R., Hebsgaard, M. B., Brand, T. B., Hofreiter, M., Bunce, M., Poinar, H. N., Dahl-Jensen, D., Johnsen, S., Steffensen, J. P., Bennike, O., Schwenninger, J.-L., Nathan, R., Armitage, S., de Hoog, C.-J., Alfimov, V., Christl, M., Beer, J., Muscheler, R., Barker, J., Sharp, M., Penkman, K. E. H., Haile, J., Taberlet, P., Gilbert, M. T. P., Casoli, A., Campani, E., and Collins, M. J.: Ancient Biomolecules from Deep Ice Cores Reveal a Forested Southern Greenland, Science, 317, 111–114, https://doi.org/10.1126/science.1141758, 2007.
Wörner, S. and Pester, M.: The Active Sulfate-Reducing Microbial Community in Littoral Sediment of Oligotrophic Lake Constance, Front. Microbiol., 10, https://doi.org/10.3389/fmicb.2019.00247, 2019.
Yin, Q. Z. and Berger, A.: Individual contribution of insolation and CO2 to the interglacial climates of the past 800,000 years, Clim. Dyn., 38, 709–724, https://doi.org/10.1007/s00382-011-1013-5, 2012.
Żarski, M., Nita, M., and Winter, H.: Nowe stanowiska interglacjalne w rejonie dolin Wilgi i Okrzejki na Wysoczyźnie Żelechowskiej (Polska południowo-wschodnia), Przegląd Geol., 53, 137–144, 2005.
Żarski, M., Hrynowiecka, A., Górecki, A., Winter, H., and Pochocka-Szwarc, K.: The maximum extent of the Odranian Glaciation (Saalian, MIS 6) in the South Podlasie Lowland (SE Poland) in the light of sites with lacustrine deposits of the Mazovian Interglacial, Acta Geol. Pol., e14–e14, https://doi.org/10.24425/agp.2024.150006, 2024.