the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Climatic impacts on mortality in pre-industrial Sweden
Tzu Tung Chen
Rodney Edvinsson
Karin Modig
Hans W. Linderholm
Fredrik Charpentier Ljungqvist
Climate variability and change, as well as extreme weather events, have notable impacts on human health and mortality. In historical times, the effect of climate on health and mortality was stronger than today, owing to factors such as poor housing and healthcare, along with the nutrition status that was meditated through climatic impacts on food production. Despite this, climatic impacts on mortality in the past remain poorly understood. This study aims to improve the understanding of climate effects on mortality using annual mortality records and meteorological data from Sweden between 1749 and 1859. The analysis includes the entire population, as well as subgroups based on sex and age. A statistically significant negative correlation was found between late winter and spring temperatures and mortality (i.e. lower temperatures equal higher mortality, and vice versa). We demonstrate that colder late winter and spring seasons were linked to higher mortality levels, not only for the same year but also for the following year. Conversely, no statistically significant associations were observed between summer or autumn temperatures and mortality, and only weak associations existed with hydroclimate. The impact of late winter and spring season temperature on mortality was most pronounced for the same year in southern Sweden and during the 19th century but stronger for the following year in central Sweden and during the 18th century. These findings call for further research, especially with respect to investigating specific diseases and additional factors contributing to the observed increase in mortality following cold late winter and spring seasons in Sweden during the late pre-industrial period.
- Article
(21538 KB) - Full-text XML
- BibTeX
- EndNote
The effects of climate change on human health and mortality have gained increasing attention in recent years in response to emerging and projected threats from anthropogenic global warming (Semenza and Menne, 2009; van Daalen et al., 2022; Romanello et al., 2022). Climate change can have direct effects on health and mortality through changes in the frequency, duration, and magnitude of exposure to temperature and hydroclimate extremes (Raymond et al., 2020; Calleja-Agius et al., 2021; Vicedo-Cabrera et al., 2021; Wu et al., 2022). Furthermore, climate change also influences the transmission of vector-borne diseases through an influence on pathogens, human susceptibility, and the abundance and distribution of certain hosts and vectors (Mills et al., 2010; Carlton et al., 2016; Rocklöv and Dubrow, 2020; Liczbińska et al., 2024). In addition, the impact of adverse climate on poorer societies, especially during historical times, affects the human nutritional status – and thus health and mortality – through its effects on agricultural productivity (Collet, 2019; Degroot et al., 2021; Ljungqvist et al., 2021). In particular, the effects of climate-triggered famine events on morbidity and mortality in the past have been comparatively well studied during recent years (e.g. Slavin, 2016; Collet and Schuh, 2018; Huhtamaa et al., 2022; Ljungqvist et al., 2024). Various other direct and indirect climatic effects on human health and mortality in historical times have been reviewed elsewhere (e.g. Diaz et al., 2001; McMichael, 2012; Robbins Schug et al., 2023).
1.1 Climate–mortality relationships: past and present
Climate and weather extremes have had considerable effects on human health and mortality patterns, primarily through their influence on food production, in pre-industrial Europe (Pfister and Wanner, 2021), especially in the Nordic countries (Huhtamaa and Ljungqvist, 2021). However, the climate–mortality relationship, and especially its geographical patterns, remains poorly quantified. Longer periods of colder temperatures tended to increase the general mortality (Galloway, 1986), and colder winters in particular increased mortality among the elderly (Galloway, 1985). The effect of cold winters on increased mortality disappeared in England already by ca. 1800, whereas the increase in mortality in response to hot summers declined throughout Europe from the late 18th century onwards (Galloway, 1994). In a more recent study, Waldinger (2022) unveiled that in England from 1538 to 1838 warmer growing seasons were associated with lower subsequent mortality, and vice versa. This effect was greater in rural areas distant from major markets.
For Sweden, Eckstein et al. (1984) reported that higher January–March temperatures reduced mortality in the 18th and 19th centuries, while the temperature effect was smaller or non-existent for the warmer months of the year. In Sweden, as was also the case in, for example, France, a statistically significant temperature effect on mortality prevailed up to ca. 1900 (Galloway, 1994). For the city of Uppsala, east-central Sweden, Schumann et al. (2013) showed that over the 1749–1859 period, a higher spring temperature decreased mortality, while a higher summer temperature increased mortality instead. Moreover, a higher spring precipitation decreased mortality, while a higher autumn precipitation increased mortality. Rocklöv et al. (2014a) conducted a similar study for the Skellefteå parish, northern coastal Sweden, for the same period. They found increased mortality in response to colder winters and springs and higher autumn precipitation particularly among children aged 3–9 but not among infants. Åström et al. (2016) found that higher temperatures, as well as higher precipitation, during the 19th and early-20th centuries were associated with lower mortality in Skellefteå but that the climate effect on mortality decreased over time. Another study has shown that higher temperatures during the summer months in Sweden over the 1880–1950 period were a significant factor in neonatal mortality rates, although the effect decreased over time and is attributable to improvements in healthcare and living conditions (Junkka et al., 2021). Perinatal mortality increased among the Swedish Sámi during cold winters (Schumann et al., 2019), and prior to ca. 1860, cold winter months increased neonatal mortality among the Sámi population (Karlsson et al., 2019). The magnitude of seasonal fluctuations in mortality rates among the elderly in Sweden has decreased substantially since at least the mid-19th century. The cohorts born in Sweden in 1800 had a risk of dying during the winter season that was almost twice the risk of dying during the summer season, while the increased risk of dying during the winter season was only about 10 % for the cohorts born in Sweden in 1900 (Ledberg, 2020).
In contemporary Europe, the elderly tend to be most vulnerable to weather-related deaths. Cold spells are linked to higher excess mortality than heat waves (van Daalen et al., 2022). Current climate change projections, however, indicate that mortality attributed to heat will start to exceed the reduction in mortality attributed to cold during the second half of the 21st century (Martínez-Solanas et al., 2021). In line with this, a study from Switzerland found that over the past decades, population ageing has attenuated the decrease in cold-related mortality and amplified heat-related mortality (de Schrijver et al., 2022). Another study by Masselot et al. (2023), analysing non-optimal temperatures and mortality in urban populations across 854 European cities, found that vulnerability to temperature increased with age for both cold and heat – although the difference is less steep for heat than it is for cold – suggesting that the effect of heat affected all ages more homogeneously. Overall, the population older than 85 years in age accounted for 60 % of the total mortality burden of extreme temperatures.
Even if age is one of the most important risk factors of extreme temperature for mortality, other factors obviously modify the effect, e.g. housing standards and access to health care (Sera et al., 2019; Bakhtsiyarava et al., 2023). Rocklöv et al. (2014b) found that the effect on mortality by heat wave duration was modified by wealth, sex, and health status. Summer temperature increases were associated with mortality increases in the group over 80 years in age, as well as with mortality increases in groups with a previous myocardial infarction and with chronic obstructive pulmonary disease in the population younger than 65 years in age. During winter, excess mortality was found particularly in men and related to the duration of cold spells for the population older than 80 years in age.
Many, but not all, causes of death display seasonal patterns. The total number of deaths follows an annual cycle, with higher mortality during the winter months in the extra-tropical Northern Hemisphere including contemporary Sweden (Statistics Sweden, 2020). Pneumonia, influenza, chronic obstructive pulmonary disease (COPD), and other respiratory diseases peak during the winter months (Lowen and Steel, 2014; Ballester et al., 2016; Achebak et al., 2023). A similar pattern, though less pronounced, is observed for cardiovascular diseases (Marti-Soler et al., 2014). Conversely, deaths from traffic accidents peak during summer months, while suicide and cancers show little or no seasonality (Rau et al., 2017). In modern Europe, the association between cold conditions and mortality in cardiovascular and respiratory diseases has weakened compared to historical times (Fonseca-Rodríguez et al., 2020, 2021). In regions characterised by warmer winters and cooler homes, a decrease in winter temperature is associated with higher increases in death rates (Eurowinter Group, 1997). In pre-industrial Europe, death from respiratory diseases showed a clear peak during the winter months, while death from gastrointestinal diseases showed a clear peak during the summer months (Buchan and Mitchell, 1875; Bradley, 1970; Lee, 1981; Wrigley and Schofield, 1981; Eckstein et al., 1984; Post, 1985; Galloway, 1987).
An additional dimension affecting the seasonality in mortality is mortality displacement or “harvesting” (Hajat et al., 2005). This refers to a short period of excess mortality (for example, due to extreme cold or heat) that could be followed by a period of lower mortality (Armstrong et al., 2017). The explanation is that mortality is displaced to occur earlier than expected among frailer individuals. A Swedish study found that a high mortality rate during winter, particularly due to respiratory and cardiovascular mortality, reduced the heat effect on mortality in the following summer (Rocklöv et al., 2008). This finding was supported by a later Australian study, which found that the estimated heat effect on mortality was generally stronger when the preceding winter mortality was low (Qiao et al., 2015). Mortality displacement is, however, complex since it may have both short-term and long-term effects and may affect different age spans and causes of death in a different way. However, a general pattern that strong excess mortality in one season is typically followed by reduced mortality in the next season seems to be true.
1.2 Malthusian demography in pre-industrial Sweden
Sweden possesses unique vital statistics at the parish level back to 1749 when a “Malthusian” demographic regime (Edvinsson, 2012), with considerable inter-annual variability in mortality in response to recurrent food crises (Larsson, 2006), still prevailed in at least large parts of the country (Dribe et al., 2017). In most other countries in Europe in possession of early vital statistics, such a Malthusian demographic regime had more or less already ended prior to the start of the systematic keeping of vital statistics. These vital statistical data have, somewhat surprisingly, not hitherto been compared – across space and time – with the instrumental climate data available in Sweden and dating back to 1722 (see, however, the pioneering study by Imhof, 1976). Such an assessment could strongly improve the understanding of climate–mortality relationship towards the end of the pre-industrial period not only in Sweden but for northern Europe as a whole.
During the Malthusian demographic regime, increased living standards tended to contribute to population growth through increased fertility and decreased mortality. In a pre-industrial Malthusian society, population growth was limited by so-called positive checks (stress factors such as famines that shortened the lifespan) and so-called preventive checks that decreased fertility (factors such as later marriages) (Galloway, 1988; Bengtsson et al., 2004; Klemp and Møller, 2016; Edvinsson, 2017). The interpretation of the Malthusian model, however, is diverse, with an emphasis on its underlying assumptions about population growth's relationship with living standards and technological innovations. As in much of Europe, there was a sharp decline in the mortality fluctuations in Sweden during the 18th century (Livi-Bacci, 2007; Edvinsson, 2017). Preventive checks on population growth first disappeared entirely in Sweden after 1870 (Bengtsson and Dribe, 2006; Dribe, 2009). Reasonably reliable estimates of crude death and birth rates exist for Sweden (within present-day borders) back to 1630 (Edvinsson, 2015). Unfortunately, these long series of crude death rates are not separated by the sex or age of the deceased. Thus, we only use the vital statistics starting in 1749 and are thus not assessing the climate–mortality relationship prior to the onset of the decline in mortality fluctuations.
Using vital statistical data from selected parishes, Larsson (2006, 2020) investigated mortality crises in Sweden during the 17th and 18th centuries. These studies have demonstrated that famines and diseases were closely intertwined during mortality crises (see also, for example, Walter and Schofield, 1989; Mokyr and Ó Gráda, 2002; Dybdahl, 2014; Ljungqvist et al., 2024). Furthermore, mortality crises during particular climate-induced shocks to food production in early modern Sweden have been investigated by Lilja (2008, 2012) and have been more comprehensively studied for the eastern part of the early modern Swedish realm in Finland (Huhtamaa and Helama, 2017; Huhtamaa, 2018; Huhtamaa et al., 2022). Spatial analysis using the vital statistics of mortality in certain diseases, chiefly dysentery, in present-day Sweden during the late 18th and early-19th centuries has been conducted by Castenbrandt (2012, 2014). The geographical distribution of malaria-attributed deaths, during peak malaria years, from 1749–1859 was assessed by Chen et al. (2021). Malaria-attributed deaths in the vital statistics for present-day Finland have been investigated by Huldén et al. (2005) and Huldén and Huldén (2009). All of these studies have revealed large inter-annual fluctuations in mortality levels.
1.3 Purpose and aim
The purpose of this article is to conduct a comprehensive examination of the impact of temperature and hydroclimate variability on mortality in present-day Sweden, considering both temporal and spatial dimensions. By analysing early vital statistics available from 1749 to 1859, we aim to build upon existing research and address the following key research questions: (1) how did climate variability during the study period correlate with mortality levels in different regions of Sweden? (2) What were the temporal patterns of mortality in relation to temperature variability in Sweden during the study period? (3) To what extent did temperature and hydroclimate variations contribute to mortality variations among specific subgroups (by age and sex) in Sweden during the studied period? These three research questions aim, in tandem, to provide insights into the relationship between climate factors and mortality, to explore regional and temporal variations, and to investigate the impact on specific population subgroups.
2.1 Mortality data
The Tabellverket dataset contains the earliest population statistics available for present-day Sweden. This dataset aggregates vital statistics from all Swedish parishes, covering the period 1749–1859, and was obtained from the Demographic Data Base (DDB) at the Centre for Demographic and Ageing Research (CEDAR) at Umeå University (Demografiska databasen, 2023). This dataset provides annual total mortality for each parish, including information on age, sex, and cause of death, albeit without specific death dates. It is important to note that the Tabellverket vital statistics have data gaps, biases, and limitations, which have been thoroughly discussed in Castenbrandt (2012). Unfortunately, prior to 1850, age was often inaccurately recorded, with discrepancies of up to 5–10 years from the actual age. This discrepancy notably introduces a bias towards an over-representation of individuals aged above 80 years (Edvinsson, 2015, p. 180). Given that only three age groups are considered in this study, these deficiencies are not expected to distort the result in any substantial way. In addition, there were instances of missing individuals and unrecorded deaths, though this remains a challenge even in contemporary population data.
In this study, we used all-cause death data (deaths that occur from any cause without specific categorisation into specific disease or condition), as well as subgroups based on sex and three age groups, namely children (0–14), adults (15–64), and the elderly (over 65). To align with the geographical coverage of malaria-related mortality conducted by Chen et al. (2021), death data from sparsely populated districts in northern Sweden were excluded. Subsequently, the remaining part of Sweden was then divided into 49 cells, each with a grid cell size of 1° × 1° (Fig. 2). A total of 7 018 694 all-cause deaths were collected from these 49 grid cells throughout the period 1749–1859. To ensure geographical accuracy, deaths from parishes that could not be precisely assigned to any of the 49 grid cells were excluded from further analysis, accounting for only 2.2 % of the total deaths (155 534 deaths out of 7 018 694 deaths) occurring between 1749 and 1859. Our study period covers 1750–1859, and information from 1749 is only included to calculate excess mortality for 1750.
Currently, no monthly mortality series exists for Sweden for the 18th century or the first part of the 19th century. Annual mortality data may not capture the seasonal nuances of mortality rates in temperate climates in which peak mortality typically occurs during the winter months (Rau et al., 2017) and roughly from November to March. This limitation makes it challenging to attribute anomalously high mortality rates during a specific year solely to the effects of exceptionally cold conditions during certain months (e.g. January–February or November–December). Despite this limitation, our study provides valuable insights into the potential connection between climate and mortality. If adverse climatic conditions have an immediate effect on mortality, we would expect correlations without time lags in our annual data. However, even in annual data, correlations with time lags may emerge if there is a delayed effect between climate conditions and mortality. Future research using more granular monthly mortality data, feasible to obtain from selected locations, could offer a more detailed understanding of these dynamics.
2.2 Climate data
The longest instrumental temperature measurements within the borders of present-day Sweden started in Uppsala in 1722 (Bergström and Moberg, 2002; Moberg et al., 2002). This meteorological record is shown in Fig. 1 as it is representative of the temperature conditions in east-central Sweden and also derives from one of the most densely populated regions in Sweden for the historical period and for the present day. Other instrumental temperature measurement series than the one in Uppsala first started later in the 18th century, and most of them are not continuous to the present day (Brönnimann et al., 2019). For the spatial analysis, we used the monthly Berkeley Earth Surface Temperatures (BEST) gridded data with a resolution of 1° × 1° since 1750 (Rohde et al., 2013a, b; Rohde and Hausfather, 2020). This gridded temperature dataset includes the Uppsala station record, in addition to many other station records. We also used the monthly gridded Palmer Drought Severity Index (PDSI) data on a 5° × 5° grid since 1750 to study the effects of hydroclimate (Briffa et al., 2009) (Fig. 2). This index integrates precipitation and temperature-driven evapotranspiration to estimate relative dryness (soil moisture) relative to the mean long-term conditions in a given region and tracks the changes in the physiological drought (Palmer, 1965; Dai et al., 2004; Wells et al., 2004; van der Schrier et al., 2011). The use of these datasets allowed us to analyse the available data overlap spanning the period from 1750 to 1859.
Our study period (1750–1859) encompasses the latter part of the generally cold Little Ice Age (Wanner et al., 2022). However, it is important to note that in certain years and even entire decades during this period, winter (Leijonhufvud et al., 2010), as well as summer (Linderholm et al., 2015; Ljungqvist et al., 2019), temperatures in Sweden were as high as those observed in the late 20th century. Hydroclimate conditions, at least during summer, mainly fluctuated within the range of observed 20th-century variations (Cook et al., 2015; Seftigen et al., 2017, 2020). The absolute temperature level, especially in spring and summer, is uncertain prior to the late 19th century due to the improper exposure of instruments, among other factors, but not with regard to the amplitude of inter-annual temperature variability (Moberg et al., 2003; Böhm et al., 2010). Precipitation measurements can be considered unreliable prior to the late 19th century in Sweden (Joelsson et al., 2024), which could introduce possible biases in the PDSI data.
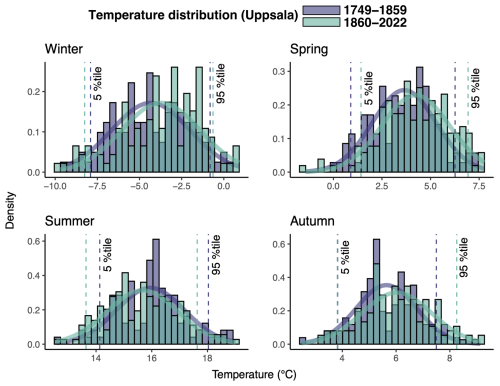
Figure 1Histograms of the distribution of absolute seasonal mean temperature for winter (December–February), spring (March–May), summer (June–August), and autumn (September–November) from the Uppsala meteorological station over the study period 1749–1859, as well as for the 1860–2022 period. The histograms are shown together with the 5 % percentiles. The Uppsala temperature record is presented because it is the longest available record in present-day Sweden covering the studied period.
2.3 Statistical methods
In this article, it is important to differentiate between the absolute number of deaths and “excess deaths”. The term excess deaths refers to the deviation from the expected number of deaths within a specific period that is attributed to particular events or circumstances (Rossen et al., 2020). Specifically, excess deaths were calculated as the difference between the actual number of deaths registered in a particular year t and the expected number of deaths for that year as estimated from a baseline derived from a quasi-Poisson regression based on the entire study period 1749–1859.
This baseline represents a long-term trend in total deaths attributable to both the strong population growth and declining mortality rates throughout this period (Fig. 3a). The time series of mortality were then correlated with monthly climate observations. Additionally, another commonly used method, also tested here, involved deriving a baseline from a linear regression using mortality or seasonal data from the preceding 5 years (see Fig. 3b). To facilitate comparisons across regions with different population sizes and age structures, we further employed the P score metric (Msemburi et al., 2023). The P score represents the percentage difference between the actual and expected number of deaths. For instance, if the expected number of deaths for a particular year t is 100, and the actual number of deaths is 120, then the excess deaths would be 20 and the P score would be 20 %, indicating that the death count is 20 % higher than expected for the year t. This metric allows a standardised measure and is calculated as follows:
While we consider the P score of excess mortality to be the most pertinent metric for this study, it has its limitations. For instance, in periods of heightened mortality, it may not reflect an increase when evaluated in relation to a trend deviation. Additionally, excess mortality does not differentiate between, for example, war-related deaths, which are certainly not directly tied to climate, and other deaths more likely to be associated with climate like malnutrition-driven diseases. In addition to this metric, we also used national-level crude death rate data (Statistics Sweden, 1999; Edvinsson, 2015) for a comparison with various baseline results (Fig. 3c). Given that crude death rate data already account for population change, we applied the quasi-Poisson regression baseline to remove the decreasing trend in mortality.
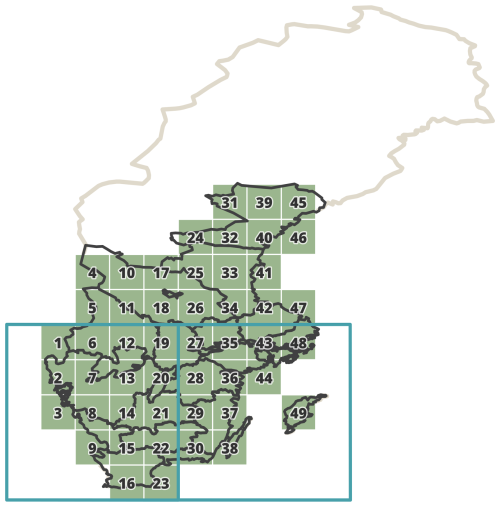
Figure 2The 49 different 1° × 1° grid cells (in green) are included for the spatial analysis between climate data and mortality in Sweden. Two 5° × 5° grid cells (in blue) are used for the Palmer Drought Severity Index (PDSI) data.
To investigate potential temporal variations in the relationship between climate and mortality, we also conducted experiments by dividing the mortality data into two separate periods, namely an earlier period spanning from 1750 to 1804 and a later period from 1805 to 1859. For each of these periods, we employed the quasi-Poisson regression baseline to calculate excess mortality. The significance levels in this article refer to p=0.05 with a two-tailed Student t test. All data integration, spatial analyses, and mapping were performed using a combination of the software FME (Safe Software Inc., 2023), QGIS (QGIS, 2023), and R (R Core Team, 2022).
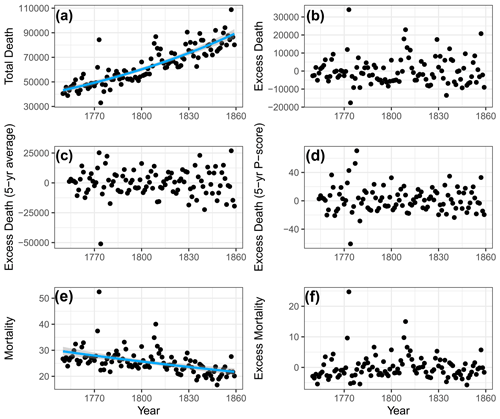
Figure 3Comparison of baselines and estimates of excess mortality. (a) Total number of deaths within 49 grid cells and a quasi-Poisson baseline (blue line). (b) Estimates of excess death. (c) Excess death using a 5-year average baseline. (d) P score of excess death using a 5-year average baseline. (e) Crude death rate and a quasi-Poisson baseline (blue line). (f) Estimates of excess mortality. Sources: the period 1630–1759 is from Edvinsson (2017), and the period 1760–1860 is from Statistics Sweden (1999).
3.1 Climate–mortality relationships on a national level
We found a statistically significant negative relationship between the late winter and spring temperature – in particular February–April temperatures – and mortality for the entire studied population for the same year and for the following year (Table 1; Fig. 4). Colder late winters and springs were associated with higher mortality, and vice versa. The spread of the data around the trend line indicates some variability in the relationship, but the overall pattern remains consistent, reflecting the statistically significant negative correlation. Despite a correlation coefficient of around −0.3, which may seem relatively weak, it is still rather noteworthy that about 10 % of the fluctuations in mortality can be explained by late winter–spring temperature variations, given the many different causes of mortality. The effect of summer and autumn temperature on mortality was non-significant – regardless of whether the temperature effect on mortality for the same or the following year is considered. Similar results were also found when using the Uppsala temperature station data instead of the gridded Berkeley temperature dataset to correlate against national-level Swedish mortality data (Table A1). In general, the Uppsala temperature data showed higher correlations with mortality compared to the Berkeley temperature dataset, which may indicate a higher reliability of the Uppsala temperature series.
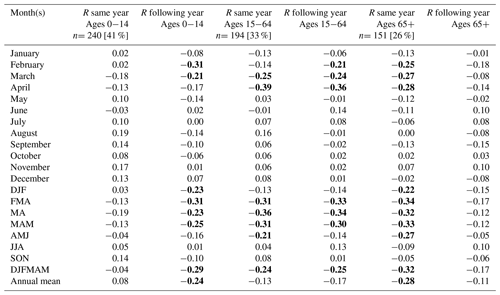
In our analysis of climate–mortality relationships categorised by sex (Table 1) and age groups (Table 3), we observed almost entirely identical mortality patterns between women and men (r=0.99 over the 1749–1859 period). Among the three age groups examined, the most statistically significant associations were found for the 15–64 age group, with correlations reaching up between April temperatures of the same year and mortality. The mortality pattern for the 15–64 age group differed from that of the over-65 age group. In the former, we observed a statistically significant negative correlation between spring temperatures and mortality during the same year and the following year. On the other hand, lower late winter and spring temperatures increased mortality among the over-65 age group during the same year only. Conversely, for the 0–14 age group, we found an increased mortality during the following year after a year with cold late winter and spring conditions.
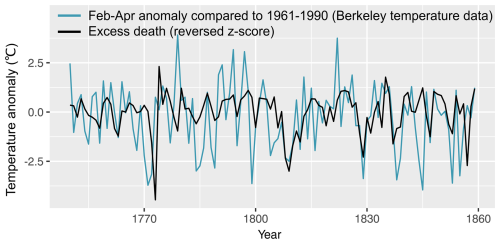
Figure 4The negative correlation () was found between February–April mean temperatures (shown here with regard to the 1961–1990 mean) using the Berkeley temperature data and standardised excess mortality of the same year (z score is shown in the opposite sign).
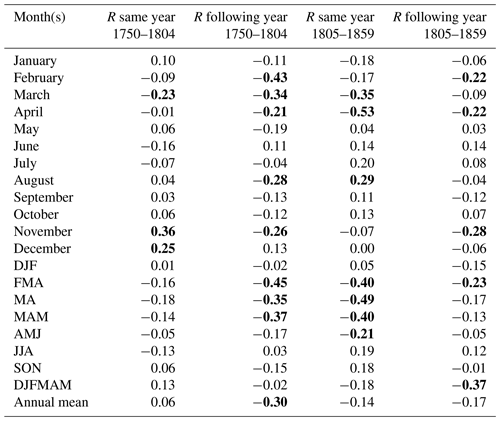
Table 1Correlation coefficient (R) between the total excess mortality (estimated by a quasi-Poisson regression) and the Berkeley temperature data (the average of all 49 grid cells) over the entire 1750–1859 period. Values which reached statistical significance (p<0.05) using a two-tailed Student t test are marked in bold. DJF is for December–February, MAM is for March–May, JJA is for June–August, and SON is for September–November.
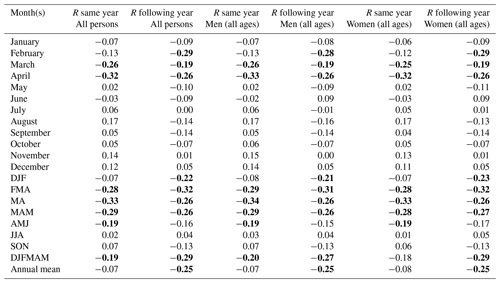
Table 2Correlation coefficient (R) between the total excess mortality (estimated by the P score using a 5-year average baseline) and the Berkeley temperature data (the average of all 49 grid cells) over the entire 1754–1859 period. Values which reached statistical significance (p<0.05) using a two-tailed Student t test are marked in bold.
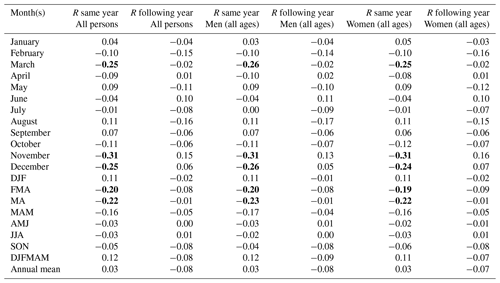
Table 3Correlation coefficient (R) between the total excess mortality (estimated by a quasi-Poisson regression) for age-specific groups and the Berkeley temperature data (the average of all 49 grid cells) over the entire 1750–1859 period. Values which reached statistical significance (p<0.05) using a two-tailed Student t test are marked in bold. n is the sample size (in millions).
3.2 Geographical patterns of the climate–mortality relationships
We investigated the geographical pattern of the temperature–mortality associations for the late winter–spring season found to exhibit the strongest such relationship. Regardless of the length of seasonal windows, we consistently observed the strongest correlations in two regions, namely southernmost Sweden for the impact of temperature on mortality during the same year and east-central Sweden (specifically the Svealand region) for the effect of temperature on mortality in the following year (Fig. 5). Time series of the excess mortality for each of the 49 grid cells is shown in Fig. A2. Maps showing the correlation coefficients with other seasonal windows with the most significant temperature–mortality relationship – namely February, March, April, March–April, and April–June – are shown in Figs. A4–A8.
The relationship between hydroclimate (soil moisture) and mortality is much weaker than the temperature–mortality associations. In terms of regional variations, the association between hydroclimate and mortality during the same year appears stronger in wetter western Sweden compared to drier eastern Sweden (Table A2). Generally, wetter conditions – especially in winter and spring – during the same year were associated with weakly increased mortality. However, this association reached statistical significance solely for January and April and only in western Sweden. Regarding the effect on mortality for the following year, wetter conditions in 1 year – especially during summer and autumn – tended to increase mortality in both western and eastern Sweden during the following year. This relationship was statistically significant for August–October in eastern Sweden and also for September and December in western Sweden (Table A2).
3.3 Changes in the climate–mortality relationships over time
Using a 31-year centred moving window correlation of the February–April mean temperature and total annual mortality in all 49 grid cells from Sweden (excess death estimated through a quasi-Poisson regression), we observed distinct patterns in the mortality response during different time periods (Fig. 6). In the 1790s, a statistically significant increase in the mortality response for the same year is detected, with a running correlation exceeding (thus explaining about 13 % of the mortality variation). This strong correlation persisted throughout most of the following period, gradually weakening after approximately 1830. The effect of February–April temperature on the mortality in the following year remained statistically significant above until 1824. However, this correlation rapidly weakened and became non-significant towards the end of the study period.
To further investigate the effect of different months on mortality, we divided the study period 1750–1859 into an earlier period (1750–1804) and a later period (1805–1859) (Table 4). The temperature effect on mortality during the same year was much stronger during the later period (1805–1859) than during the earlier period (1750–1804). The correlation reached for April and for March–April during the 1805–1859 period. This means that about 25 % of the variation in mortality is explained. Conversely, the temperature effect on the following year's mortality was stronger during the earlier period for most monthly and seasonal windows and decreased from to −0.23 from the earlier to the later period. Moreover, we found that higher November and December temperatures resulted in significantly higher mortality during the 1750–1804 period, but this effect was not observed during the later period. In addition, a statistically significant increase in mortality in response to higher August temperatures during the same year was only observed during the 1805–1859 period. In summary, the temperature effect on mortality during the same year was stronger during the latter period (as shown in Fig. 6). Interestingly, the temperature effect on mortality during the same year in Scania (the southernmost part of Sweden) weakened over time and shifted to eastern Svealand (around Stockholm). However, for the temperature effect on mortality in the following year, this effect was observed in large regions but gradually became insignificant over time (Fig. 7).
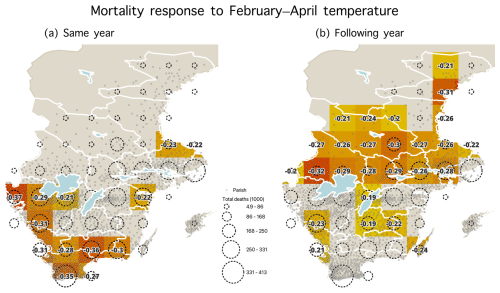
Figure 5Statistically significant correlation coefficients (p<0.05) during the 1750–1859 period between February–April temperature and mortality for (a) the same year and (b) the following year. Stronger correlations are indicated by darker colours on the maps. Parishes are represented by dots, and dashed circles represent the number of total deaths (1000) from parishes within each grid cell.
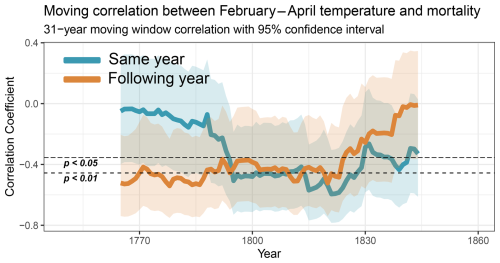
Figure 6Moving correlation using a 31-year centred moving window with a 95 % confidence interval (shaded areas) between February–April mean temperature and mortality (excess mortality is estimated by a quasi-Poisson regression). Dashed lines indicate the statistical significance level at p<0.05 and p<0.01.
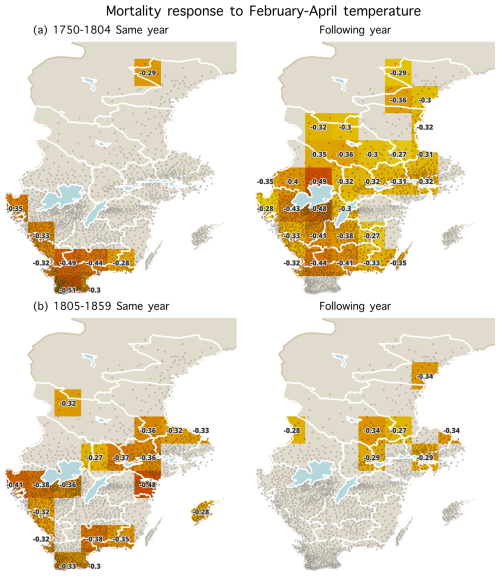
Figure 7Statistically significant correlation coefficients (p<0.05) between February–April average temperatures and mortality (all persons) during the same year and the following year for the periods (a) 1750–1804 and (b) 1805–1859, respectively. Stronger correlations are indicated by darker colours on the maps. Parishes are represented by dots.
3.4 Extreme cold-related and warm-related mortality
We further analysed the geographical patterns of the relationship between February–April temperature and mortality for the same year and the following year by especially focusing on the six coldest (<5th percentile) and six warmest (>95th percentile) February–April seasons between 1750 and 1859 (Fig. 8). The coldest February–March seasons during this period occurred in 1845, 1771, 1799, 1853, 1838, and 1829, while the warmest such seasons were observed in 1779, 1822, 1794, 1797, 1750, and 1791 (also shown in Fig. 4). The results reveal a diffuse pattern when compared to the long-term relationship shown in Fig. 5. In the southernmost region of Sweden, Scania, where the long-term February–April temperatures had the strongest long-term effect on the mortality during the same year, this effect was not particularly pronounced during either the extremely cold or warm years. However, for the effect on mortality during the following year, some extremely cold February–April seasons (1772, 1800, and 1839) exhibited similar patterns in central Sweden (Fig. 8a) as the persistent temperature–mortality relationship. Similarly, some extremely warm February–April seasons (1823, 1798, and 1751) were linked to lower mortality (Fig. 8b). Unexpectedly, the warmest February–April season, 1779, was followed by higher mortality.
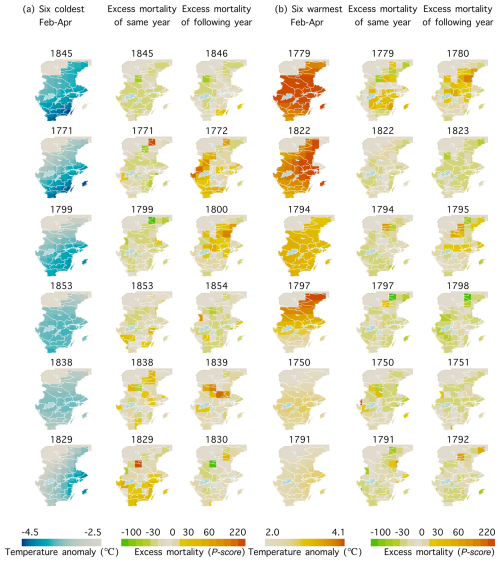
Figure 8Spatial patterns of mortality (here we used the P score in order to compare the relative increase in excess mortality across regions with different population sizes) on the (a) six coldest (<5th percentile) and (b) six warmest (>95th percentile) February–April seasons from 1750 to 1859 for the same year and the following year. The years are arranged in descending order from the coldest/warmest (maps near the top) to the least cold or warm (maps near the bottom). Temperature anomaly values are relative to the 1961–1990 mean derived from Berkeley temperature data.
4.1 Climate–mortality relationships: time lags, geographical patterns, and subgroups
We have observed geographical differences in the winter and spring temperature effects on mortality between the southwestern part of Sweden and central Sweden. The temperature effect on mortality during the same year was much stronger in the former region, whereas the temperature effect on mortality in the following year was much stronger in the latter region (Sect. 3.2). Galloway (1994) found a statistically significant mortality increase in response to colder winters in Sweden, and to a lesser extent to colder springs, whereas we found a stronger spring temperature effect than winter temperature effect on mortality. However, we did not detect a statistically significant mortality increase in response to warmer summers, contrary to the findings of Galloway (1994). These discrepancies may be attributed to methodological differences as we considered the correlation coefficient between time series, while Galloway (1994) considered lag sum responses. Similar to our study, Eckstein et al. (1984) found little influence of summer temperatures on pre-industrial Swedish mortality. However, Schumann et al. (2013) reported increased mortality in Uppsala due to higher summer temperatures. This could presumably be explained by the fact that the Uppsala region experiences higher summer temperatures, particularly higher maximum temperatures, compared to most of Sweden (Wastenson et al., 1995). While our findings also demonstrate a small influence of hydroclimatic conditions on mortality in late pre-industrial Sweden (Sect. 3.2), it is important to note that this phenomenon is not only weak but also exhibits geographical and temporal variations. The hydroclimatic signal is so weak, although partly statistically significant, that we leave it out of the discussion. However, we emphasise the importance of recognising that multiple climatic factors contribute to variations in mortality rates in the late pre-industrial era in Sweden, in particular during specific individual extreme years.
Although the absence of a temperature effect on the following year's mortality in southwesternmost Sweden can be explained by the region's agriculture being less sensitive to a late onset of the growing season (Skoglund, 2022, 2024), one could have expected central Sweden to also display an effect of the cold winters and springs on the same year's mortality. The increased mortality observed the year following colder late winters and springs in central Sweden can almost certainly be attributed to a reduced food supply resulting from adverse climatic effects on agriculture (Edvinsson et al., 2009; Dribe et al., 2017). However, this should not, in principle, preclude an impact of temperature on mortality during the same year as well. It is highly plausible that during the 18th century, when the mortality rate was generally higher, the impact of cold winters and springs on increased mortality was less discernible compared to the 19th century when the mortality rate had decreased. With a lower mortality rate, it can be expected that even smaller increases in mortality in response to extreme climatic conditions would appear more prominently amidst the overall larger variability in mortality. One likely explanation for the weakening relationship after about 1825 is the improved living conditions, including better nutrition, during the 19th century. Overall mortality, mainly from infectious diseases, decreased, and fluctuations in death rates were reduced. For example, smallpox was nearly eradicated with the mandatory vaccination introduced in 1816 (Sköld, 1996).
Whereas men and women were equally affected by changes in temperature, the impact of temperature on mortality differed between age groups. Among the 0–14 age group, mortality increased during the following year after a year with colder spring and winter conditions (Sect. 3). This can probably be largely attributed to the effects of spring temperatures on food production (Edvinsson et al., 2009; Holopainen et al., 2012) and, thus, on nutrition levels (Ljungqvist et al., 2024). It is likely that this especially affected infant mortality, which accounted for much of the mortality within the 0–14 age group in 18th- and 19th-century Sweden (Statistics Sweden, 1969). For the age group 15–64, the negative association between late winter and spring temperature and mortality can be attributed to several factors. Cold winter and spring conditions during the pre-industrial era have been associated with the increased spread of respiratory and contagious diseases (Galloway, 1994). This is likely due to indoor crowding and decreased immune system resistance to respiratory infections in cold temperatures (Eurowinter Group, 1997). Such respiratory deaths occur rapidly and contribute to higher mortality within the same year. In addition, the increased mortality during the year following cold late winters and springs can be explained by adverse effects on agriculture triggering increased malnutrition. Moreover, a noteworthy negative association was observed between the same year's winter and spring temperatures and mortality among the elderly (aged 65 and older). Cold temperatures could have directly impacted the health of older individuals, making them more susceptible to respiratory infections, cardiovascular events, and other cold-related health risks (Fonseca-Rodríguez et al., 2020). For example, influenza mortality tends to increase with cold and dry conditions (Lowen et al., 2007; Lowen and Steel, 2014). An area to further investigate in future research is the potential mortality displacement (“harvesting effect”) among the elderly during cold years with higher mortality that could then be presumed to be followed by lower mortality and weaker effects of exposure in the following year(s).
4.2 The effects of climate-induced harvest variations on mortality
Late winter and early-spring temperatures have been found to have played a critical role in grain production in much of the main agricultural districts in south–central Sweden (Edvinsson et al., 2009; Holopainen et al., 2012). Warm winters and springs tended to increase harvests as long as late spring and summer droughts did not suppress them (for the latter, see Ljungqvist et al., 2023). Furthermore, cold late winters and springs had an adverse effect on dairy production in Sweden (Utterström, 1955); this was also seen in regions with comparatively milder climates such as the British Isles (Costello et al., 2023) and Germany (Baten, 2001). Thus, it is likely that all, or most, of the negative relationship between late winter and spring temperature and the following year's mortality rate was due to climate effects on food availability. In fact, the relationship between temperature and the mortality of the following year, which is strongest in central Sweden and prior to the 1820s, can hardly be explained in any way other than through changes in food availability. Its geographical pattern, being largely restricted to the Svealand region, needs to be further investigated in terms of the climate sensitivity of the agriculture in the region and the societal resilience to food shortage in this region compared to southernmost Sweden. The mortality associated with malnutrition in the 18th and 19th centuries was primarily driven by disease outbreaks (Mokyr and Ó Gráda, 2002; Larsson, 2006, 2020; Ljungqvist et al., 2024). However, to better understand the extent to which climate-related mortality fluctuations can be attributed to starvation or malnutrition, further research is needed to investigate the causes of death recorded in the vital statistics from that period.
The following year's mortality response to climate variability is thus presumed to mainly operate by affecting the nutritional situation through the intermediary of the harvest outcome and, to a lesser extent, livestock mortality. We acknowledge that the exclusion of harvest data, as a central intermediate stage between climate and mortality, constitutes a major limitation in the present study. However, we leave the complex issue of harvest–mortality relationships to further studies due to several issues compromising the reliability and representativeness of 18th- and 19th-century Swedish grain harvest data. First, the yield ratio series were typically collected from individual farms, which may not accurately reflect entire regions and may not even be locally representative, as they tended to be biased towards the most prosperous farms or manors (Slicher van Bath, 1963). Second, tithes are frequently used as an indicator of harvest volume (Leijonhufvud, 2001); however, tithes in Sweden captured only 25 % to 50 % of the actual harvests, with some being fixed amounts. During the study period, only small gradually decreasing portions of Sweden still paid taxes in tithes that reflected the actual harvest variations (Hallberg et al., 2016). Third, harvest data do not necessarily translate into actual food availability, as grain and other food sources could be, and were, imported from other regions (Edvinsson, 2012) or from abroad (Åmark, 1915). The third point, however, is of limited relevance here as the observed association between climatic and following year's mortality must reasonably be interpreted as a result of local-to-regional harvest outcomes.
At present, a reconstruction of Swedish harvests (Edvinsson, 2009) is available for our study period only until 1820 and, as a national average, has a limited explanatory value for studying regional harvest–mortality relationships. This harvest reconstruction, however, indicates an increasing per capita harvest from about 1810, with setbacks already occurring by the 1790s, signifying increased food security. The per capita harvests, when including potatoes, were about 15 %–20 % higher around 1820 than they had been about 30 years earlier (Edvinsson, 2009). This agrees well with our finding that the relationship between winter–spring temperature and the following year's mortality became non-significant during the 1820s. Furthermore, we note that this concurs with the findings by Larsson (2006). In his work with mortality data from selected parishes, Larsson (2006) also interpreted increased mortality during the winter–spring season as being indicative of malnutrition due to the previous year's harvest failures and found that these seasonal mortality peaks declined over time as a result of an improved food supply.
4.3 Methodological considerations
When estimating excess mortality, or any change in mortality beyond normal fluctuations that is attributed to an external factor, the choice of comparison period or baseline is crucial (Sect. 2.3). This baseline represents a reference for estimating the expected mortality level in the absence of the variable of interest. While the concept of excess mortality has been studied extensively, the COVID-19 pandemic has further highlighted the importance of this methodology. The challenge lies not only in properly estimating the baseline but also in finding a method that allows for meaningful comparisons between regions or countries. This complexity arises from the fact that different countries, or regions, may have experienced varying trends in mortality and population dynamics. Different methodologies have been developed and evaluated, ranging from simple strategies such as comparing death counts from the previous year to advanced regression models that aim to account for changes in both mortality levels and population characteristics.
In this study, we tested two different methods for estimating baseline, or expected, mortality, namely a baseline fitted from a quasi-Poisson regression baseline and a baseline derived from a linear regression based on the mortality during the past 5 years. The use of a 5-year average, or trend, as a baseline for studying excess mortality during the COVID-19 pandemic was a common and straightforward approach (Barnard et al., 2022; Nepomuceno et al., 2022; Wang et al., 2022; Levitt et al., 2023). It can be effective when mortality rates are relatively stable over time. However, in our study period characterised by significant fluctuations in mortality, where sudden events like wars or epidemics can have a substantial but temporary impact on annual mortality, the 5-year baseline becomes a sensitive reference period. An outlier event can significantly influence the baseline, leading to either an overestimation or an underestimation of excess mortality. Moreover, this method does not account for any long-term trends in mortality, which can be a limitation. This issue is more pronounced in modern times, which is when mortality generally shows an improvement with every consecutive year. In our study period, the year-to-year variability in mortality posed the main challenge. To address this issue, we adopted a reference period that encompassed the full study period using the quasi-Poisson regression model. This approach allowed us to account for the long-term trends and mitigate the impact of inter-annual fluctuations on mortality. As a sensitivity analysis, we also stratified the time period into two segments to explore any potential temporal variations in the climate–mortality relationship. Interestingly, the results from this stratified analysis aligned closely with those obtained from the full study period, confirming the robustness of our findings.
As a result, the excess mortality using a quasi-Poisson regression baseline (Table 1) demonstrated stronger correlations with temperature changes compared to the 5-year approach (Table 2), thus aligning with findings from other studies comparing different baseline estimation methods. While the methods may yield different levels of excess, the overall patterns tend to remain consistent (Nepomuceno et al., 2022; Levitt et al., 2023). The P score, however, proved to be a more suitable measure for comparing the relative increase in excess mortality across regions with different population sizes and possibly different age structures. This is due to the inherent tendency for larger population sizes and older age groups to have higher excess death counts. In addition, the comparison of excess crude death rates and excess deaths using a quasi-Poisson regression baseline (Table A3) revealed similar outcomes. Both measures showed statistically significant correlations with temperature, suggesting that excess death using a quasi-Poisson regression baseline can effectively serve as a measure for excess mortality.
There are several limitations to consider in this study. First, the use of annual resolved mortality data restricted the possibility of examining the seasonality of climate-related mortality, which would have provided valuable insights into the climate–mortality relationship. Second, our analysis primarily focused on the individual effects of temperature and, to a limited extent, hydroclimate variables and did not use classified climate types such as hot/wet, hot/dry, cold/wet, or cold/dry. An inclusive consideration of climate variables would provide an effective assessment of the risks associated with different diseases. Moving forward, future research could gain a deeper understanding by exploring the climate–mortality relationship in different time periods, geographical regions, and, in particular, cause-specific mortalities. In addition, the integration of a more comprehensive dataset, including factors such as land use and socioeconomic indicators, would provide a more nuanced understanding of region-specific mortality patterns and their relationship with climate. Incorporating data on grain harvests and regional grain import could enable a more thorough examination of the extent to which food availability was mediated through climate impacts. Furthermore, a full understanding of the causes of climate-related mortality is only possible by studying changes in the cause of death in relation to various climate parameters. The data from Tabellverket would, in principle, allow for an investigation. However, it would require a major research undertaking.
Our study has revealed a statistically significant association between temperature and mortality in Sweden during the period 1750–1859. Colder temperatures in late winter and spring were found to elevate mortality rates during the same year, with this impact extending also into the following year. This effect of February, March, and April temperatures was particularly large for mortality during the same and the following year. Exploring geographical differences in the temperature–mortality relationship, we found that the southernmost regions experienced the greatest impact of temperature on mortality during the same year. Conversely, central Sweden exhibited the strongest temperature effect on mortality in the following year. This suggests that in central and northern Sweden, the influence of temperature on mortality was primarily attributed to the adverse effects of cold conditions on food production, while this was not the case in southern Sweden. To establish causation definitively, future studies should incorporate data on both regional harvests and monthly mortality.
Child mortality was notably influenced by the effect of cold late winter and spring conditions in the previous year, likely associated with malnutrition. In contrast, mortality among the elderly was more immediate and potentially linked to cold-related diseases that affected health within the same year. It is noteworthy that the temperature–mortality relationship changed over time, showing a diminishing impact after the 1820s, especially for the following year's mortality. This indicates improved food and nutrition security during that period. Further research is needed to refine and expand upon these findings. This includes a more in-depth exploration of the underlying factors that contributed to the variation in the climate–mortality relationship patterns between the southern and central regions of Sweden, as well as a detailed analysis of the specific diseases and other causes of death associated with the season- and age-related mortality.
Table A1Correlations of excess mortality (estimated by a quasi-Poisson regression) against Uppsala temperature station data (Moberg and Bergström, 1997) over the period 1749–1859. Values which reached statistical significance (p<0.05) using a two-tailed Student t test are marked in bold.
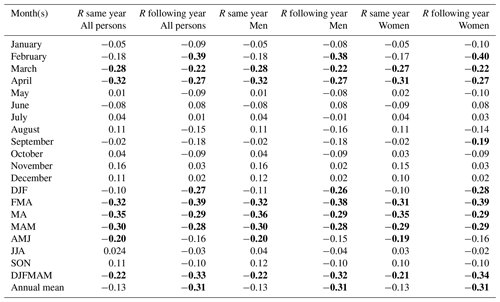
Table A2Correlations of excess mortality (estimated by a quasi-Poisson regression) with PDSI data over the entire period (1750–1859). Sweden is divided following longitude 15° E into a western and an eastern part. Values which reached statistical significance (p<0.05) using a two-tailed Student t test are marked in bold.
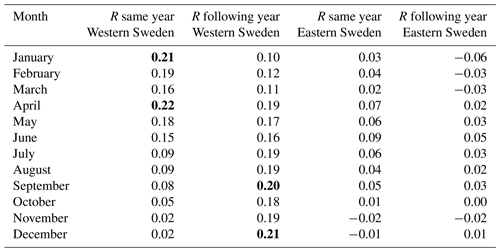
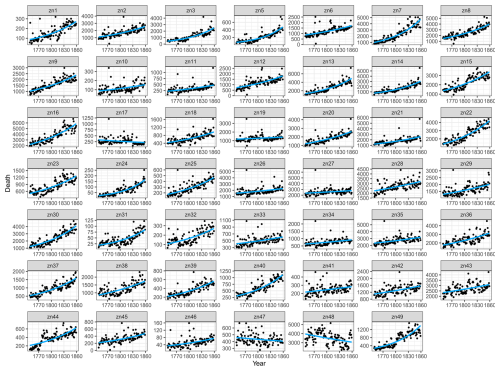
Figure A1Respective baselines estimated by a quasi-Poisson regression. No parish was located within grid cell no. 4; hence, no data are presented.
Figure A2Excess all-cause death using a quasi-Poisson baseline for all age groups of each of the 49 grid cells during 1749–1859. Excess deaths were estimated by the difference between actual deaths and the baseline estimated for each grid cell. No parish was located within grid cell no. 4; hence, no data are presented.
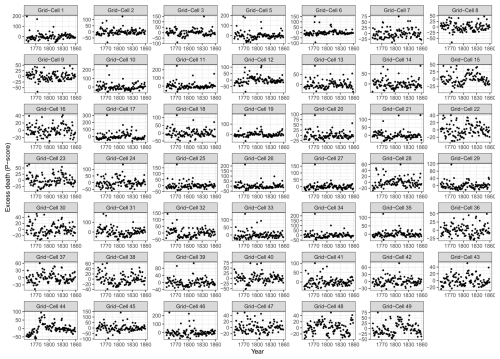
Figure A3Excess all-cause death as the P score, using a quasi-Poisson baseline for all age groups of each of the 49 grid cells during 1749–1859. No parish was located within grid cell no. 4; hence, no data are presented.
Table A3Comparison of correlations of excess mortality (estimated by a quasi-Poisson regression) and excess crude death rate for all persons with the Berkeley temperature data from 49 grid cells during 1750–1859. Values which reached statistical significance (p<0.05) using a two-tailed Student t test are marked in bold.
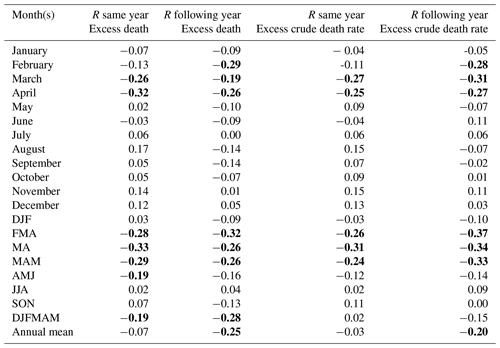
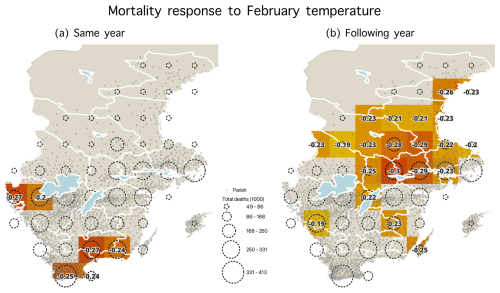
Figure A4Statistically significant correlations (p<0.05) during the 1750–1859 period between February temperature and mortality for (a) the same year and (b) the following year. Parishes are shown with dots, and dashed circles represent the number of total deaths (1000) from parishes within each grid cell.
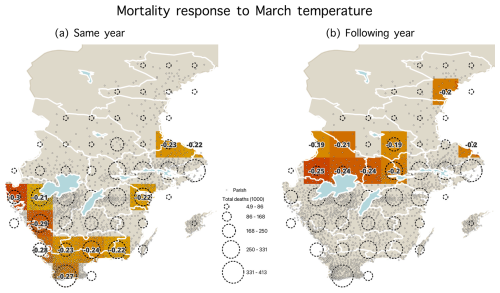
Figure A5Statistically significant correlations (p<0.05) during the 1750–1859 period between the March temperature period and mortality for (a) the same year and (b) the following year. Parishes are shown with dots, and dashed circles represent the number of total deaths (1000) from parishes within each grid cell.
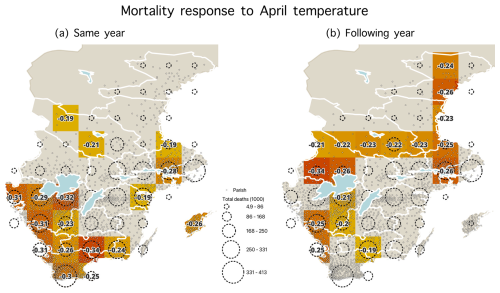
Figure A6Statistically significant correlations (p<0.05) during the 1750–1859 period between April temperature and mortality for (a) the same year and (b) the following year. Parishes are shown with dots, and dashed circles represent the number of total deaths (1000) from parishes within each grid cell.
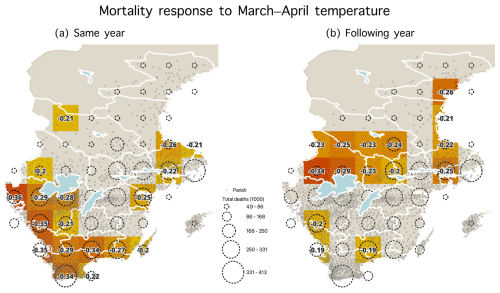
Figure A7Statistically significant correlations (p<0.05) during the 1750–1859 period between the March–April temperatures and mortality for (a) the same year and (b) the following year. Parishes are shown with dots, and dashed circles represent the number of total deaths (1000) from parishes within each grid cell.
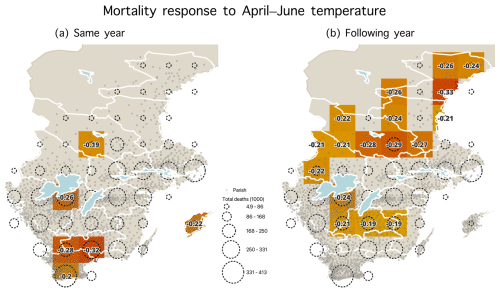
Figure A8Statistically significant correlations (p<0.05) during the 1750–1859 period between the April–June temperatures and mortality for (a) the same year and (b) the following year. Parishes are shown with dots, and dashed circles represent the number of total deaths (1000) from parishes within each grid cell.
We have used FME (Safe Software Inc., 2023), QGIS (QGIS, 2023), and R (R Core Team, 2022) platforms to program the analysis codes used in this work. The R code and FME workflow are available from the corresponding author upon reasonable request.
The entire population mortality data can be accessed through the open-data SHiPS platform (https://doi.org/10.17197/U23003, Demografiska databasen, 2023) at Demografiska databasen, the Centre for Demographic and Ageing Research (CEDAR), Umeå University, Sweden https://doi.org/10.17197/U23003. However, the age-specific mortality data used in the current article are not publicly available and require a license. These data can be made available upon reasonable request and with the permission of CEDAR. The Berkeley Earth Surface Temperatures (BEST; Rohde et al., 2013a, b; Rohde and Hausfather, 2020) is licensed under Creative Commons (CC BY-NC 4.0) International for non-commercial use only and is available from https://berkeleyearth.org/data/ (Rohde and Hausfather, 2020). The Uppsala temperature station data (Bergström and Moberg, 2002) are freely available from the Swedish Meteorological and Hydrological Institute at https://www.smhi.se/data/meteorologi/temperatur/uppsalas-temperaturserie-1.2855. The PDSI data from Briffa et al. (2009) are freely available from the Climatic Research Unit at the University of East Anglia at https://crudata.uea.ac.uk/cru/data/drought/europe.
TTC helped to conceive and design the study, collected and integrated the data, conducted the statistical and geostatistical analyses, created figures, and wrote part of the text. RE provided expertise on the pre-industrial demography in Sweden. KM provided expertise regarding the calculation of excess mortality and modern climate–mortality relationships. HWL provided valuable input on the study design and provided climatological expertise. FCL conceived and designed the study and wrote much of the text.
At least one of the (co-)authors is a member of the editorial board of Climate of the Past. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
Fredrik Charpentier Ljungqvist acknowledges a Visiting Researcher stay at the Freiburg Institute for Advanced Studies (FRIAS), which allowed him time to finalise the work with this article. The authors thank the two anonymous reviewers and Bertil Forsberg at Umeå University, whose useful comments helped improve this article.
Tzu Tung Chen was supported by the Swedish Research Council Formas (grant no. 2017-01161) and by the Bolin Centre for Climate Research, Stockholm University (grant no. RT3. BC: 30002632). Hans W. Linderholm was funded by the Swedish Research Council (grant no. 2021-03886). Fredrik Charpentier Ljungqvist was supported by the project “Adapting to climate change in the northern Baltic Sea region, AD 1500–1900” funded by Marianne and Marcus Wallenberg Foundation (grant no. MMW 2022-0114), and he conducted the work with this article as a Pro Futura Scientia XIII Fellow funded by the Swedish Collegium for Advanced Study through Riksbankens Jubileumsfond. Open-access publication funding for this article was provided by Stockholm University.
This paper was edited by Chantal Camenisch and reviewed by two anonymous referees.
Achebak, H., Garcia-Aymerich, J., Rey, G., Chen, Z., Méndez-Turrubiates, R. F., and Ballester, J.: Ambient temperature and seasonal variation in inpatient mortality from respiratory diseases: a retrospective observational study, Lancet Reg. Health Eur., 35, 100757, https://doi.org/10.1016/j.lanepe.2023.100757, 2023. a
Åmark, K.: Spannmålshandel och spannmålspolitik i Sverige 1719–1830, Stockholms högskola, Stockholm, 387 pp., 1915. a
Armstrong, B., Bell, M. L., de Sousa Zanotti Stagliorio Coelho, M., Leon Guo, Y.-L., Guo, Y., Goodman, P., Hashizume, M., Honda, Y., Kim, H., Lavigne, E., Michelozzi, P., Hilario, P., Saldiva, N., Schwartz, J., Scortichini, M., Sera, F., Tobias, A., Tong, S., Wu, C.-f., Zanobetti, A., Zeka, A., and Gasparrini, A.: Longer-term impact of high and low temperature on mortality: an international study to clarify length of mortality displacement, Environ. Health Perspect., 125, 107009, https://doi.org/10.1289/EHP1756, 2017. a
Åström, D. O., Edvinsson, S., Hondula, D., Rocklöv, J., and Schumann, B.: On the association between weather variability and total and cause-specific mortality before and during industrialization in Sweden, Demographic Res., 35, 991–1010, https://doi.org/10.4054/DemRes.2016.35.33, 2016. a
Bakhtsiyarava, M., Schinasi, L. H., Sánchez, B. N., Dronova, I., Kephart, J. L., Ju, Y., Gouveia, N., Caiaffa, W. T., O'Neill, M. S., Yamada, G., Arunachalam, S., Diez-Roux, A. V., and Rodríguez, D. A.: Modification of temperature-related human mortality by area-level socioeconomic and demographic characteristics in Latin American cities, Soc. Sci. Med., 317, 115526, https://doi.org/10.1016/j.socscimed.2022.115526, 2023. a
Ballester, J., Rodó, X., Robine, J.-M., and Herrmann, F. R.: European seasonal mortality and influenza incidence due to winter temperature variability, Nat. Clim. Change., 6, 927–930, https://doi.org/10.1038/nclimate3070, 2016. a
Barnard, S., Chiavenna, C., Fox, S., Charlett, A., Waller, Z., Andrews, N., Goldblatt, P., Burton, P., and De Angelis, D.: Methods for modelling excess mortality across England during the COVID-19 pandemic, Stat. Methods Med. Res., 31, 1790–1802, https://doi.org/10.1177/09622802211046384, 2022. a
Baten, J.: Climate, grain production and nutritional status in southern Germany during the XVIIIth century, J. Eur. Econ. Hist., 30, 9–47, 2001. a
Bengtsson, T. and Dribe, M.: Deliberate control in a natural fertility population: Southern Sweden, 1766–1864, Demography, 43, 727–746, https://doi.org/10.1353/dem.2006.0030, 2006. a
Bengtsson, T., Campbell, C., and Lee, J. Z.: Life under Pressure: Mortality and Living Standards in Europe and Asia, MIT Press, Cambridge, MA, https://doi.org/10.7551/mitpress/4227.001.0001, 2004. a
Bergström, H. and Moberg, A.: Daily air temperature and pressure series for Uppsala (1722–1998), Clim. Change, 53, 213–252, https://doi.org/10.1023/A:1014983229213, 2002. a, b
Böhm, R., Jones, P. D., Hiebl, J., Frank, D., Brunetti, M., and Maugeri, M.: The early instrumental warm-bias: a solution for long central European temperature series 1760–2007, Clim. Change, 101, 41–67, https://doi.org/10.1007/s10584-009-9649-4, 2010. a
Bradley, L.: An enquiry into seasonality in baptisms, marriages, and burials, Local Popul. Stud., 4, 21–40, http://www.localpopulationstudies.org.uk/PDF/LPS5/LPS5_1970_18-35.pdf (last access: 17 January 2025), 1970. a
Briffa, K., van der Schrier, G., and Jones, P.: Wet and dry summers in Europe since 1750: evidence of increasing drought, Int. J. Climatol., 29, 1894–1905, https://doi.org/10.1002/joc.1836, 2009. a, b
Brönnimann, S., Allan, R., Ashcroft, L., Baer, S., Barriendos, M., Brázdil, R., Brugnara, Y., Brunet, M., Brunetti, M., Chimani, B., Cornes, R., Domínguez-Castro, F., Filipiak, J., Founda, D., Herrera, R. G., Gergis, J., Grab, S., Hannak, L., Huhtamaa, H., Jacobsen, K. S., Jones, P., Jourdain, S., Kiss, A., Lin, K. E., Lorrey, A., Lundstad, E., Luterbacher, J., Mauelshagen, F., Maugeri, M., Moberg, A., Neukom, R., Nicholson, S., Noone, S., Nordli, Ø., Ólafsdóttir, K. B., Pearce, P. R., Pfister, L., Pribyl, K., Przybylak, R., Pudmenzky, C., Rasol, D., Reichenbach, D., Řezníčková, L., Rodrigo, F. S., Rohr, C., Skrynyk, O., Slonosky, V., Thorne, P., Valente, M. A., Vaquero, J. M., Westcottt, N. E., Williamson, F., and Wyszyński, P.: Unlocking pre-1850 instrumental meteorological records: A global inventory, Bull. Am. Meteorol. Soc., 100, ES389–ES413, https://doi.org/10.1175/BAMS-D-19-0040.1, 2019. a
Buchan, A. and Mitchell, A.: On the influence of weather on mortality from different diseases and at different ages, J. Scott. Meteorol. Soc., 4, 187–265, 1875. a
Calleja-Agius, J., England, K., and Calleja, N.: The effect of global warming on mortality, Early Hum. Dev., 155, 105222, https://doi.org/10.1016/j.earlhumdev.2020.105222, 2021. a
Carlton, E. J., Woster, A. P., DeWitt, P., Goldstein, R. S., and Levy, K.: A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases, Int. J. Epidemiol., 45, 117–130, https://doi.org/10.1093/ije/dyv296, 2016. a
Castenbrandt, H.: Rödsot i Sverige 1750–1900: En sjukdoms demografiska och medicinska historia, Ph.D. thesis, University of Gothenburg, Gothenburg, 234 pp., http://hdl.handle.net/2077/30195 (last access: 17 January 2025), 2012. a, b
Castenbrandt, H.: A forgotten plague: dysentery in Sweden, 1750–1900, Scand. J. Hist., 39, 612–639, https://doi.org/10.1080/03468755.2014.953199, 2014. a
Chen, T. T., Ljungqvist, F. C., Castenbrandt, H., Hildebrandt, F., Ingholt, M. M., Hesson, J. C., Ankarklev, J., Seftigen, K., and Linderholm, H. W.: The spatiotemporal distribution of historical malaria cases in Sweden: a climatic perspective, Malar. J., 20, 1–14, https://doi.org/10.1186/s12936-021-03744-9, 2021. a, b
Collet, D.: Die doppelte Katastrophe: Klima und Kultur in der europäischen Hungerkrise 1770–1772, 466 pp., Vandenhoeck & Ruprecht, Göttingen, ISBN 978-3-525-35592-3, 2019. a
Collet, D. and Schuh, M. (Eds.): Famines During the `Little Ice Age' (1300–1800): Socio-natural Entanglements in Premodern Societies, Springer, Berlin/Heidelberg, https://doi.org/10.1007/978-3-319-54337-6, 2018. a
Cook, E. R., Seager, R., Kushnir, Y., Briffa, K. R., Büntgen, U., Frank, D., Krusic, P. J., Tegel, W., van der Schrier, G., Andreu-Hayles, L., Baillie, M., Baittinger, C., Bleicher, N., Bonde, N., Brown, D., Carrer, M., Cooper, R., Čufar, K., Dittmar, C., Esper, J., Griggs, C., Gunnarson, B., Günther, B., Gutierrez, E., Haneca, K., Helama, S., Herzig, F., Heussner, K.-U., Hofmann, J., Janda, P., Kontic, R., Köse, N., Kyncl, T., Levanič, T., Linderholm, H., Manning, S., Melvin, T. M., Miles, D., Neuwirth, B., Nicolussi, K., Nola, P., Panayotov, M., Popa, I., Rothe, A., Seftigen, K., Seim, A., Svarva, H., Svoboda, M., Thun, T., Timonen, M., Touchan, R., Trotsiuk, V., Trouet, V., Walder, F., Ważny, T., Wilson, R., and Zang, C.: Old World megadroughts and pluvials during the Common Era, Sci. Adv., 1, e1500561, https://doi.org/10.1126/sciadv.1500561, 2015. a
Costello, E., Kearney, K., and Gearey, B.: Adapting to the Little Ice Age in pastoral regions: An interdisciplinary approach to climate history in north-west Europe, Hist. Methods, 56, 77–96, https://doi.org/10.1080/01615440.2022.2156958, 2023. a
Dai, A., Trenberth, K. E., and Qian, T.: A global dataset of Palmer Drought Severity Index for 1870–2002: Relationship with soil moisture and effects of surface warming, J. Hydrometeorol., 5, 1117–1130, https://doi.org/10.1175/JHM-386.1, 2004. a
Degroot, D., Anchukaitis, K., Bauch, M., Burnham, J., Carnegy, F., Cui, J., de Luna, K., Guzowski, P., Hambrecht, G., Huhtamaa, H., Izdebski, A., Kleemann, K., Moesswilde, E., Neupane, N., Newfield, T., Pei, Q., Xoplaki, E., and Zappia, N.: Towards a rigorous understanding of societal responses to climate change, Nature, 591, 539–550, https://doi.org/10.1038/s41586-021-03190-2, 2021. a
Demografiska databasen: Centre for Demographic and Ageing Research (CEDAR), U23003 [data set], https://doi.org/10.17197/U23003, 2023. a, b
de Schrijver, E., Bundo, M., Ragettli, M. S., Sera, F., Gasparrini, A., Franco, O. H., and Vicedo-Cabrera, A. M.: Nationwide analysis of the heat- and cold-related mortality trends in Switzerland between 1969 and 2017: the role of population aging, Environ. Health Perspect., 130, 037001, https://doi.org/10.1289/EHP9835, 2022. a
Diaz, H. F., Kovats, R. S., McMichael, A. J., and Nicholls, N.: Climate and human health linkages on multiple timescales, in: History and Climate: Memories of the Future?, edited by: Jones, P. D., Ogilvie, A. E. J., Davies, T. D., and Briffa, K. R., 267–289 pp., Springer, Berlin/Heidelberg, https://doi.org/10.1007/978-1-4757-3365-5_13, 2001. a
Dribe, M.: Demand and supply factors in the fertility transition: A county-level analysis of age-specific marital fertility in Sweden, 1880–1930, Eur. Rev. Econ. Hist., 13, 65–94, https://doi.org/10.1017/S1361491608002372, 2009. a
Dribe, M., Olsson, M., and Svensson, P.: Nordic Europe, in: Famine in European history, edited by: Alfani, G. and Ó Gráda, C., 185–211 pp., Cambridge University Press, Cambridge, https://doi.org/10.1017/9781316841235.009, 2017. a, b
Dybdahl, A.: Klimatiske sjokk, uår, sykdom og demografiske kriser i Trøndelag på 1600- og 1700-tallet, Hist. Tidskr., 93, 243–275, https://doi.org/10.18261/ISSN1504-2944-2014-02-05, 2014. a
Eckstein, Z., Schultz, T. P., and Wolpin, K. I.: Short-run fluctuations in fertility and mortality in pre-industrial Sweden, Eur. Econ. Rev., 26, 295–317, https://doi.org/10.1016/0014-2921(84)90093-X, 1984. a, b, c
Edvinsson, R.: Swedish harvests, 1665–1820: Early modern growth in the periphery of European economy, Scand. Econ. Hist. Rev., 57, 2–25, https://doi.org/10.1080/03585520802631592, 2009. a, b
Edvinsson, R.: Harvests and grain prices in Sweden, 1665–1870, Agric. Hist. Rev., 60, 1–18, https://www.jstor.org/stable/23317128 (last access: 17 January 2025), 2012. a, b
Edvinsson, R.: Recalculating Swedish pre-census demographic data: Was there acceleration in early modern population growth?, Cliometrica, 9, 167–191, https://doi.org/10.1007/s11698-014-0112-z, 2015. a, b, c
Edvinsson, R., Leijonhufvud, L., and Söderberg, J.: Väder, skördar och priser i Sverige, in: Agrarhistoria på många sätt: 28 studier om människan och jorden. Festskrift till Janken Myrdal på hans 60-årsdag, edited by: Liljewall, B., Flygare, I. A., Lange, U., Ljunggren, L., and Söderberg, J., 115–136 pp., The Royal Swedish Academy of Agriculture and Forestry, Stockholm, ISBN 9789185205912, 2009. a, b, c
Edvinsson, R. B.: The response of vital rates to harvest fluctuations in pre-industrial Sweden, Cliometrica, 11, 245–268, https://doi.org/10.1007/s11698-016-0144-7, 2017. a, b, c
Eurowinter Group: Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe, Lancet, 349, 1341–1346, https://doi.org/10.1016/S0140-6736(96)12338-2, 1997. a, b
Fonseca-Rodríguez, O., Sheridan, S. C., Lundevaller, E. H., and Schumann, B.: Hot and cold weather based on the spatial synoptic classification and cause-specific mortality in Sweden: a time-stratified case-crossover study, Int. J. Biometeorol., 64, 1435–1449, https://doi.org/10.1007/s00484-020-01921-0, 2020. a, b
Fonseca-Rodríguez, O., Sheridan, S. C., Lundevaller, E. H., and Schumann, B.: Effect of extreme hot and cold weather on cause-specific hospitalizations in Sweden: A time series analysis, Environ. Res., 193, 110535, https://doi.org/10.1016/j.envres.2020.110535, 2021. a
Galloway, P. R.: Annual variations in deaths by age, deaths by cause, prices, and weather in London 1670 to 1830, Popul. Stud., 39, 487–505, https://doi.org/10.1080/0032472031000141646, 1985. a
Galloway, P. R.: Long-term fluctuations in climate and population in the preindustrial era, Popul. Dev. Rev., 12, 1–24, https://doi.org/10.2307/1973349, 1986. a
Galloway, P. R.: Population, Prices, and Weather in Preindustrial Europe, Ph.D. thesis, University of California, Berkeley, 954 pp., 1987. a
Galloway, P. R.: Basic patterns in annual variations in fertility, nuptiality, mortality, and prices in pre-industrial Europe, Popul. Stud., 42, 275–303, https://doi.org/10.1080/0032472031000143366, 1988. a
Galloway, P. R.: Secular changes in the short-term preventive, positive, and temperature checks to population growth in Europe, 1460 to 1909, Clim. Change, 26, 3–63, https://doi.org/10.1007/BF01094008, 1994. a, b, c, d, e, f
Hajat, S., Armstrong, B. G., Gouveia, N., and Wilkinson, P.: Mortality displacement of heat-related deaths: a comparison of Delhi, São Paulo, and London, Epidemiology, 16, 613–620, https://doi.org/10.1097/01.ede.0000164559.41092.2a, 2005. a
Hallberg, E., Leijonhufvud, L., Linde, M., and Andersson Palm, L.: Skördar i Sverige före agrarrevolutionen: Statistisk undersökning av det rörliga tiondet fr.o.m. 1665: Introduktion till databaser, Department of Historical Studies, University of Gothenburg, Gothenburg, https://doi.org/10.5878/002873, 2016. a
Holopainen, J., Rickard, I. J., and Helama, S.: Climatic signatures in crops and grain prices in 19th-century Sweden, Holocene, 22, 939–945, https://doi.org/10.1177/0959683611434220, 2012. a, b
Huhtamaa, H.: Combining written and tree-ring evidence to trace past food crises: A case study from Finland, in: Famines During the 'Little Ice Age' (1300–1800), edited by: Collet, D. and Schuh, M., 43–66 pp., Springer, Berlin/Heidelberg, https://doi.org/10.1007/978-3-319-54337-6_3, 2018. a
Huhtamaa, H. and Helama, S.: Distant impact: tropical volcanic eruptions and climate-driven agricultural crises in seventeenth-century Ostrobothnia, Finland, J. Hist. Geogr., 57, 40–51, https://doi.org/10.1016/j.jhg.2017.05.011, 2017. a
Huhtamaa, H. and Ljungqvist, F. C.: Climate in Nordic historical research – a research review and future perspectives, Scand. J. Hist., 46, 665–695, https://doi.org/10.1080/03468755.2021.1929455, 2021. a
Huhtamaa, H., Stoffel, M., and Corona, C.: Recession or resilience? Long-range socioeconomic consequences of the 17th century volcanic eruptions in northern Fennoscandia, Clim. Past, 18, 2077–2092, https://doi.org/10.5194/cp-18-2077-2022, 2022. a, b
Huldén, L. and Huldén, L.: The decline of malaria in Finland – the impact of the vector and social variables, Malar. J., 8, 94, https://doi.org/10.1186/1475-2875-8-94, 2009. a
Huldén, L., Huldén, L., and Heliövaara, K.: Endemic malaria: an “indoor” disease in northern Europe. Historical data analysed, Malar. J., 4, 1–13, https://doi.org/10.1186/1475-2875-4-19, 2005. a
Imhof, A. E.: Aspekte der Bevölkerungsentwicklung in den nordischen Ländern: 1720–1750, Francke, Giessen, Vol. 1–2, 1122 pp., ISBN 3772011853, 1976. a
Joelsson, L. M. T., Södling, J., Kjellström, E., and Josefsson, W.: Comparison of historical and modern precipitation measurement techniques in Sweden, Idöjárás, 128, 195–218, https://doi.org/10.28974/idojaras.2024.2.4, 2024. a
Junkka, J., Karlsson, L., Lundevaller, E., and Schumann, B.: Climate vulnerability of Swedish newborns: Gender differences and time trends of temperature-related neonatal mortality, 1880–1950, Environ. Res., 192, 110400, https://doi.org/10.1016/j.envres.2020.110400, 2021. a
Karlsson, L., Lundevaller, E., and Schumann, B.: The association between cold extremes and neonatal mortality in Swedish Sápmi from 1800 to 1895, Glob. Health Action, 12, 1623609, https://doi.org/10.1080/16549716.2019.1623609, 2019. a
Klemp, M. and Møller, N. F.: Post-Malthusian dynamics in pre-industrial Scandinavia, Scand. J. Econ., 118, 841–867, https://doi.org/10.1111/sjoe.12155, 2016. a
Larsson, D.: Den dolda transitionen: Om ett demografiskt brytningsskede i det tidiga 1700-talets Sverige, Ph.D. thesis, University of Gothenburg, Gothenburg, 258 pp., ISBN 9188614573, http://hdl.handle.net/2077/16842 (last access: 17 January 2025), 2006. a, b, c, d, e
Larsson, D.: Diseases in early modern Sweden: A parish-level study 1631–1775, Scand. J. Hist., 45, 407–432, https://doi.org/10.1080/03468755.2019.1659178, 2020. a, b
Ledberg, A.: A large decrease in the magnitude of seasonal fluctuations in mortality among elderly explains part of the increase in longevity in Sweden during 20th century, BMC Public Health, 20, 1–10, https://doi.org/10.1186/s12889-020-09749-4, 2020. a
Lee, R. D.: Short-term variation: Vital rates, prices and weather, in: The Population History of England, edited by: Wrigley, E. A. and Schofield, R. S., 356–401 pp., Cambridge University Press, Cambridge, 1981. a
Leijonhufvud, L.: Grain Tithes and Manorial Yields in Early Modern Sweden: Trends and Patterns of Production and Productivity c. 1540–1680, Ph.D. thesis, Swedish University of Agricultural Sciences, Ulltuna, 359 pp., ISBN 9157658293, 2001. a
Leijonhufvud, L., Wilson, R., Moberg, A., Söderberg, J., Retsö, D., and Söderlind, U.: Five centuries of Stockholm winter/spring temperatures reconstructed from documentary evidence and instrumental observations, Clim. Change, 101, 109–141, https://doi.org/10.1007/s10584-009-9650-y, 2010. a
Levitt, M., Zonta, F., and Ioannidis, J.: Excess death estimates from multiverse analysis in 2009–2021, Eur. J. Epidemiol., 38, 1129–1139, https://doi.org/10.1007/s10654-023-00998-2, 2023. a, b
Liczbińska, G., Vögele, J. P., and Brabec, M.: Climate and disease in historical urban space: evidence from 19th century Poznań, Poland, Clim. Past, 20, 137–150, https://doi.org/10.5194/cp-20-137-2024, 2024. a
Lilja, S.: Klimatet, döden och makten – 1690-talets klimatkris, in: Leva vid Östersjöns kust: en antologi om naturförutsättningar och resursutnyttjande på båda sidor av Östersjön ca 800–1800, edited by: Lilja, S., 23–79 pp., Södertörns högskola, Stockholm, http://sh.diva-portal.org/smash/get/diva2:213729/FULLTEXT01.pdf (last access: 17 January 2025), 2008. a
Lilja, S.: Klimat och skördar ca 1530–1820, in: Fiske, jordbruk och klimat i Östersjöregionen under förmodern tid: Projektet Förmoderna kustmiljöer, edited by: Lilja, S., 59–119 pp., Södertörns högskola, Stockholm, https://www.diva-portal.org/smash/get/diva2:477376/FULLTEXT01.pdf (last access: 17 January 2025), 2012. a
Linderholm, H. W., Björklund, J., Seftigen, K., Gunnarson, B. E., and Fuentes, M.: Fennoscandia revisited: a spatially improved tree-ring reconstruction of summer temperatures for the last 900 years, Clim. Dynam., 45, 933–947, https://doi.org/10.1007/s00382-014-2328-9, 2015. a
Livi-Bacci, M.: A Concise History of World Population, Wiley-Blackwell, Cambridge, Mass., 279 pp., https://doi.org/10.1002/9781119406822, 2007. a
Ljungqvist, F. C., Seim, A., Krusic, P. J., González-Rouco, J. F., Werner, J. P., Cook, E. R., Zorita, E., Luterbacher, J., Xoplaki, E., Destouni, G., García-Bustamante, E., Aguilar, C. A. M., Seftigen, K., Wang, J., Gagen, M. H., Esper, J., Solomina, O., Fleitmann, D., and Büntgen, U.: European warm-season temperature and hydroclimate since 850 CE, Environ. Res. Lett., 14, 084015, https://doi.org/10.1088/1748-9326/ab2c7e, 2019. a
Ljungqvist, F. C., Seim, A., and Huhtamaa, H.: Climate and society in European history, Wiley Interdisciplin. Rev.: Clim. Change, 12, e691, https://doi.org/10.1002/wcc.691, 2021. a
Ljungqvist, F. C., Christiansen, B., Esper, J., Huhtamaa, H., Leijonhufvud, L., Pfister, C., Seim, A., Skoglund, M. K., and Thejll, P.: Climatic signatures in early modern European grain harvest yields, Clim. Past, 19, 2463–2491, https://doi.org/10.5194/cp-19-2463-2023, 2023. a
Ljungqvist, F. C., Seim, A., and Collet, D.: Famines in medieval and early modern Europe – connecting climate and society, Wiley Interdisciplin. Rev.: Clim. Change, 15, e859, https://doi.org/10.1002/wcc.859, 2024. a, b, c, d
Lowen, A. C. and Steel, J.: Roles of humidity and temperature in shaping influenza seasonality, J. Virol., 88, 7692–7695, https://doi.org/10.1128/JVI.03544-13, 2014. a, b
Lowen, A. C., Mubareka, S., Steel, J., and Palese, P.: Influenza virus transmission is dependent on relative humidity and temperature, PLoS Pathogens, 3, e151, https://doi.org/10.1371/journal.ppat.0030151, 2007. a
Marti-Soler, H., Gonseth, S., Gubelmann, C., Stringhini, S., Bovet, P., Chen, P.-C., Wojtyniak, B., Paccaud, F., Tsai, D.-H., Zdrojewski, T., and Marques-Vidal, P.: Seasonal variation of overall and cardiovascular mortality: A study in 19 countries from different geographic locations, PloS One, 9, e113500, https://doi.org/10.1371/journal.pone.0113500, 2014. a
Martínez-Solanas, È., Quijal-Zamorano, M., Achebak, H., Petrova, D., Robine, J.-M., Herrmann, F. R., Rodó, X., and Ballester, J.: Projections of temperature-attributable mortality in Europe: a time series analysis of 147 contiguous regions in 16 countries, Lancet Planet. Health, 5, e446–e454, https://doi.org/10.1016/S2542-5196(21)00150-9, 2021. a
Masselot, P., Mistry, M., Vanoli, J., Schneider, R., Iungman, T., Garcia-Leon, D., Ciscar, J.-C., Feyen, L., Orru, H., Urban, A., Breitner, S., Huber, V., Schneider, A., Samoli, E., Stafoggia, M., de’Donato Francesca, Rao, S., Armstrong, B., Nieuwenhuijsen, M., Vicedo-Cabrera, A. M., and Gasparrini, A.: Excess mortality attributed to heat and cold: a health impact assessment study in 854 cities in Europe, Lancet Planet. Health, 7, e271–e281, https://doi.org/10.1016/S2542-5196(23)00023-2, 2023. a
McMichael, A. J.: Insights from past millennia into climatic impacts on human health and survival, P. Natl. Acad. Sci. USA, 109, 4730–4737, https://doi.org/10.1073/pnas.1120177109, 2012. a
Mills, J. N., Gage, K. L., and Khan, A. S.: Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan, Environ. Health Perspect., 118, 1507–1514, https://doi.org/10.1289/ehp.0901389, 2010. a
Moberg, A. and Bergström, H.: Homogenization of Swedish temperature data. Part III: The long temperature records from Uppsala and Stockholm, Int. J. Climatol., 17, 667–699, https://doi.org/10.1002/(SICI)1097-0088(19970615)17:7<667::AID-JOC115>3.0.CO;2-J, 1997. a
Moberg, A., Bergström, H., Krigsman, J. R., and Svanered, O.: Daily air temperature and pressure series for Stockholm (1756–1998), Clim. Change, 53, 171–212, https://doi.org/10.1007/978-94-010-0371-1_7, 2002. a
Moberg, A., Alexandersson, H., Bergström, H., and Jones, P. D.: Were southern Swedish summer temperatures before 1860 as warm as measured?, Int. J. Climatol., 23, 1495–1521, https://doi.org/10.1002/joc.945, 2003. a
Mokyr, J. and Ó Gráda, C.: What do people die of during famines: the Great Irish Famine in comparative perspective, Eur. Rev. Econ. Hist., 6, 339–363, https://doi.org/10.1017/S1361491602000163, 2002. a, b
Msemburi, W., Karlinsky, A., Knutson, V., Aleshin-Guendel, S., Chatterji, S., and Wakefield, J.: The WHO estimates of excess mortality associated with the COVID-19 pandemic, Nature, 613, 130–137, https://doi.org/10.1038/s41586-022-05522-2, 2023. a
Nepomuceno, M. R., Klimkin, I., Jdanov, D. A., Alustiza-Galarza, A., and Shkolnikov, V. M.: Sensitivity analysis of excess mortality due to the COVID-19 pandemic, Popul. Dev. Rev., 48, 279–302, https://doi.org/10.1111/padr.12475, 2022. a, b
Palmer, W. C.: Meteorological Drought, US Department of Commerce, Weather Bureau, Washington D.C., 1965. a
Pfister, C. and Wanner, H.: Climate and Society in Europe: The Last Thousand Years, Bern: Haupt Verlag, 397 pp., ISBN 978-3-258-08234-9, 2021. a
Post, J. D.: Food Shortage, Climatic Variability, and Epidemic Disease in Preindustrial Europe: The Mortality Peak in the Early 1740s, Cornell University Press, Ithaca, 303 pp., ISBN 0-8014-1773-2, 1985. a
QGIS: QGIS Geographic Information System. Open Source Geospatial Foundation Project, https://www.qgis.org/ (last access: 17 January 2025), 2023. a, b
Qiao, Z., Guo, Y., Yu, W., and Tong, S.: Assessment of short-and long-term mortality displacement in heat-related deaths in Brisbane, Australia, 1996–2004, Environ. Health Perspect., 123, 766–772, https://doi.org/10.1289/ehp.1307606, 2015. a
R Core Team: R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (last access: 17 January 2025), 2022. a, b
Rau, R., Bohk-Ewald, C., Muszyńska, M. M., and Vaupel, J. W.: Seasonality of causes of death, in: Visualizing Mortality Dynamics in the Lexis Diagram, edited by: Rau, R., Bohk-Ewald, C., Muszyńska, M. M., and Vaupel, J. W., 99–122 pp., Springer, Berlin/Heidelberg, https://doi.org/10.1007/978-3-319-64820-0_9, 2017. a, b
Raymond, C., Matthews, T., and Horton, R. M.: The emergence of heat and humidity too severe for human tolerance, Sci. Adv., 6, eaaw1838, https://doi.org/10.1126/sciadv.aaw1838, 2020. a
Robbins Schug, G., Buikstra, J. E., DeWitte, S. N., Baker, B. J., Berger, E., Buzon, M. R., Davies-Barrett, A. M., Goldstein, L., Grauer, A. L., Gregoricka, L. A., Halcrow, S. E., Knudson, K. J., Larsen, C. S., Martin, D. L., Nystrom, K. C., Perry, M. A., Roberts, C. A., Santos, A. L., Stojanowski, C. M., Suby, J. A., Temple, D. H., Tung, T. A., Vlok, M., Watson-Glen, T., and Zakrzewski, S. R.: Climate change, human health, and resilience in the Holocene, P. Natl. Acad. Sci. USA, 120, e2209472120, https://doi.org/10.1073/pnas.2209472120, 2023. a
Rocklöv, J. and Dubrow, R.: Climate change: an enduring challenge for vector-borne disease prevention and control, Nat. Immunol., 21, 479–483, https://doi.org/10.1038/s41590-020-0648-y, 2020. a
Rocklöv, J., Edvinsson, S., Arnqvist, P., De Luna, S. S., and Schumann, B.: Association of seasonal climate variability and age-specific mortality in northern Sweden before the onset of industrialization, Int. J. Environ. Res. Public Health., 11, 6940–6954, https://doi.org/10.3390/ijerph110706940, 2014a. a
Rocklöv, J., Forsberg, B., Ebi, K., and Bellander, T.: Susceptibility to mortality related to temperature and heat and cold wave duration in the population of Stockholm County, Sweden, Glob. Health Action, 7, 22737, https://doi.org/10.3402/gha.v7.22737, 2014b. a
Rocklöv, J. P., Forsberg, B., and Meister, K.: Winter mortality modifies the heat-mortality association the following summer, Epidemiology, 19, S87–S88, https://doi.org/10.1183/09031936.00037808, 2008. a
Rohde, R., Muller, R., Jacobsen, R., Muller, E., Perlmutter, S., Rosenfeld, A., Wurtele, J., Groom, D., and Wickham, C.: A new estimate of the average Earth surface land temperature spanning 1753 to 2011, Geoinform. Geostat.: An Overview, 1, 1–7, https://doi.org/10.4172/2327-4581.1000101, 2013a. a
Rohde, R., Muller, R., Jacobsen, R., Perlmutter, S., Rosenfeld, A., Wurtele, J., Curry, J., Wickham, C., and Mosher, S.: Berkeley Earth temperature averaging process, Geoinformatics Geostatistics: An Overview, 1, 20–100, https://doi.org/10.4172/2327-4581.1000103, 2013b. a
Rohde, R. A. and Hausfather, Z.: The Berkeley Earth Land/ Ocean Temperature Record, Earth Syst. Sci. Data, 12, 3469–3479, https://doi.org/10.5194/essd-12-3469-2020, 2020 (data available at: https://berkeleyearth.org/data/, last access: 17 January). a, b
Romanello, M., Di Napoli, C., Drummond, P., Green, C., Kennard, H., Lampard, P., Scamman, D., Arnell, N., Ayeb-Karlsson, S., and Ford, L. B.: The 2022 report of the Lancet Countdown on health and climate change: health at the mercy of fossil fuels, Lancet, 400, 1619–1654, https://doi.org/10.1016/S0140-6736(22)01540-9, 2022. a
Rossen, L. M., Branum, A. M., Ahmad, F. B., Sutton, P., and Anderson, R. N.: Excess deaths associated with COVID-19, by age and race and ethnicity – United States, January 26–October 3, 2020, Morb. Mortal. Wkly. Rep., 69, 1522, https://doi.org/10.15585/mmwr.mm6942e2, 2020. a
Safe Software Inc.: Feature Manipulation Engine, https://www.safe.com/ (last access: 17 January 2025), 2023. a, b
Schumann, B., Edvinsson, S., Evengård, B., and Rocklöv, J.: The influence of seasonal climate variability on mortality in pre-industrial Sweden, Glob. Health Action, 6, 20153, https://doi.org/10.3402/gha.v6i0.20153, 2013. a, b
Schumann, B., Häggström Lundevaller, E., and Karlsson, L.: Weather extremes and perinatal mortality – Seasonal and ethnic differences in northern Sweden, 1800–1895, PLoS One, 14, e0223538, https://doi.org/10.1371/journal.pone.0223538, 2019. a
Seftigen, K., Goosse, H., Klein, F., and Chen, D.: Hydroclimate variability in Scandinavia over the last millennium – insights from a climate model–proxy data comparison, Clim. Past, 13, 1831–1850, https://doi.org/10.5194/cp-13-1831-2017, 2017. a
Seftigen, K., Fuentes, M., Ljungqvist, F. C., and Björklund, J.: Using Blue Intensity from drought-sensitive Pinus sylvestris in Fennoscandia to improve reconstruction of past hydroclimate variability, Clim. Dynam., 55, 579–594, https://doi.org/10.1007/s00382-020-05287-2, 2020. a
Semenza, J. C. and Menne, B.: Climate change and infectious diseases in Europe, Lancet Infect. Dis., 9, 365–375, https://doi.org/10.1016/S1473-3099(09)70104-5, 2009. a
Sera, F., Armstrong, B., Tobias, A., Vicedo-Cabrera, A. M., Åström, C., Bell, M. L., Chen, B.-Y., de Sousa Zanotti Stagliorio Coelho, M., Matus Correa, P., Cruz, J. C., Dang, T. N., Hurtado-Diaz, M., Do Van, D., Forsberg, B., Guo, Y. L., Guo, Y., Hashizume, M., Honda, Y., Iñiguez, C., Jaakkola, J. J. K., Kan, H., Kim, H., Lavigne, E., Michelozzi, P., Ortega, N. V., Osorio, S., Pascal, M., Ragettli, M. S., Ryti, N. R. I., Saldiva, P. H. N., Schwartz, J., Scortichini, M., Seposo, X., Tong, S., Zanobetti, A., and Gasparrini, A.: How urban characteristics affect vulnerability to heat and cold: a multi-country analysis, Int. J. Epidemiol., 48, 1101–1112, https://doi.org/10.1093/ije/dyz008, 2019. a
Skoglund, M. K.: Climate variability and grain production in Scania, 1702–1911, Clim. Past, 18, 405–433, https://doi.org/10.5194/cp-18-405-2022, 2022. a
Skoglund, M. K.: The impact of drought on northern European pre-industrial agriculture, Holocene, 34, 120–135, https://doi.org/10.1177/09596836231200431, 2024. a
Sköld, P.: From inoculation to vaccination: Smallpox in Sweden in the eighteenth and nineteenth centuries, Popul. Stud., 50, 247–262, https://doi.org/10.1080/0032472031000149336, 1996. a
Slavin, P.: Climate and famines: A historical reassessment, Wiley Interdisciplin. Rev.: Clim. Change, 7, 433–447, https://doi.org/10.1002/wcc.395, 2016. a
Slicher van Bath, B. H.: Yield Ratios, 1810–1820, Afdeling Agrarische Geschiedenis, Universiteit Wageningen, Wageningen, 264 pp., https://edepot.wur.nl/296371 (last access: 17 January 2025), 1963. a
Statistics Sweden: Historisk statistik för Sverige Del 1. Befolkning 1720–1967, Stockholm: SCB, 177 pp., http://hdl.handle.net/2077/854 (last access: 17 January 2025), 1969. a
Statistics Sweden: Population Development in Sweden in a 250-year Perspective, Statistics Sweden, Stockholm, 165 pp., ISBN 91-618-1021-5, http://hdl.handle.net/2077/857 (last access: 17 January 2025), 1999. a, b
Statistics Sweden: Information About the Statistics on Mortality, Statistics Sweden, Örebro, 7 pp., https://www.scb.se/contentassets/1d234c96211e427997250c52de572504/text-om-statistiken-over-doda_en.pdf (last access: 17 January 2025), 2020. a
Utterström, G.: Climatic fluctuations and population problems in early modern history, Scand. Econ. Hist. Rev., 3, 3–47, https://doi.org/10.1080/03585522.1955.10411467, 1955. a
van Daalen, K. R., Romanello, M., Rocklöv, J., Semenza, J. C., Tonne, C., Markandya, A., Dasandi, N., Jankin, S., Achebak, H., Ballester, J., Bechara, H., Callaghan, M. W., Chambers, J., Dasgupta, S., Drummond, P., Farooq, Z., Gasparyan, O., Gonzalez-Reviriego, N., Hamilton, I., Hänninen, R., Kazmierczak, A., Kendrovski, V., Kennard, H., Kiesewetter, G., Lloyd, S. J., Lotto Batista, M., Martinez-Urtaza, J., Milà, C., Minx, J. C., Nieuwenhuijsen, M., Palamarchuk, J., Quijal-Zamorano, M., Robinson, E. J. Z., Scamman, D., Schmoll, O., Sewe, M. O., Sjödin, H., Sofiev, M., Solaraju-Murali, B., Springmann, M., Triñanes, J., Anto, J. M., Nilsson, M., and Lowe, R.: The 2022 Europe report of the Lancet Countdown on health and climate change: towards a climate resilient future, Lancet Public Health, 7, e942–e965, https://doi.org/10.1016/S2468-2667(22)00197-9, 2022. a, b
van der Schrier, G., Jones, P., and Briffa, K.: The sensitivity of the PDSI to the Thornthwaite and Penman-Monteith parameterizations for potential evapotranspiration, J. Geophys. Res.-Atmos., 116, D03106, https://doi.org/10.1029/2010JD015001, 2011. a
Vicedo-Cabrera, A. M., Scovronick, N., Sera, F., Royé, D., Schneider, R., Tobias, A., Astrom, C., Guo, Y., Honda, Y., Hondula, D. M., Abrutzky, R., Tong, S., Coelho, M. d. S. Z. S., Saldiva, P. H. N., Lavigne, E., Correa, P. M., Ortega, N. V., Kan, H., Osorio, S., Kyselý, J., Urban, A., Orru, H., Indermitte, E., Jaakkola, J. J. K., Ryti, N., Pascal, M., Schneider, A., Katsouyanni, K., Samoli, E., Mayvaneh, F., Entezari, A., Goodman, P., Zeka, A., Michelozzi, P., de’Donato, F., Hashizume, M., Alahmad, B., Diaz, M. H., Valencia, C. D. L. C., Overcenco, A., Houthuijs, D., Ameling, C., Rao, S., Di Ruscio, F., Carrasco-Escobar, G., Seposo, X., Silva, S., Madureira, J., Holobaca, I. H., Fratianni, S., Acquaotta, F., Kim, H., Lee, W., Iniguez, C., Forsberg, B., Ragettli, M. S., Guo, Y. L. L., Chen, B. Y., Li, S., Armstrong, B., Aleman, A., Zanobetti, A., Schwartz, J., Dang, T. N., Dung, D. V., Gillett, N., Haines, A., Mengel, M., Huber, V., and Gasparrini, A.: The burden of heat-related mortality attributable to recent human-induced climate change, Nat. Clim. Change, 11, 492–500, https://doi.org/10.1038/s41558-021-01058-x, 2021. a
Waldinger, M.: The economic effects of long-term climate change: evidence from the Little Ice Age, J. Polit. Econ., 130, 2275–2314, https://doi.org/10.1086/720393, 2022. a
Walter, J. and Schofield, R.: Famine, disease and crisis mortality in early modern society, in: Famine, Disease and the Social Order in Early Modern Society, edited by: Walter, J., Schofield, R., and Appleby, A. B., 1–74 pp., Cambridge University Press, Cambridge, https://doi.org/10.1017/CBO9780511599637.003, 1989. a
Wang, H., Paulson, K. R., Pease, S. A., Watson, S., Comfort, H., Zheng, P., Aravkin, A. Y., Bisignano, C., Barber, R. M., Alam, T., Fuller, J. E., May, E. A., Jones, D. P., Frisch, M. E., Abbafati, C., Adolph, C., Allorant, A., Amlag, J. O., Bang-Jensen, B., Bertolacci, G. J., Bloom, S. S., Carter, A., Castro, E., Chakrabarti, S., Chattopadhyay, J., Cogen, R. M., Collins, J. K., Cooperrider, K., Dai, X., Dangel, W. J., Daoud, F., Dapper, C., Deen, A., Duncan, B. B., Erickson, M., Ewald, S. B., Fedosseeva, T., Ferrari, A. J., Frostad, J. J., Fullman, N., Gallagher, J., Gamkrelidze, A., Guo, G., He, J., Helak, M., Henry, N. J., Hulland, E. N., Huntley, B. M., Kereselidze, M., Lazzar-Atwood, A., LeGrand, K. E., Lindstrom, A., Linebarger, E., Lotufo, P. A., Lozano, R., Magistro, B., Malta, D. C., Månsson, J., Mantilla Herrera, A. M., Marinho, F., Mirkuzie, A. H., Misganaw, A. T., Monasta, L., Naik, P., Nomura, S., O'Brien, E. G., O'Halloran, J. K., Olana, L. T., Ostroff, S. M., Penberthy, L., Reiner Jr, R. C., Reinke, G., Ribeiro, A. L. P., Santomauro, D. F., Schmidt, M. I., Shaw, D. H., Sheena, B. S., Sholokhov, A., Skhvitaridze, N., Sorensen, R. J. D., Spurlock, E. E., Syailendrawati, R., Topor-Madry, R., Troeger, C. E., Walcott, R., Walker, A., Wiysonge, C. S., Worku, N. A., Zigler, B., Pigott, D. M., Naghavi, M., Mokdad, A. H., Lim, S. S., Hay, S. I., Gakidou, E., and Murray, C. J. L.: Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21, Lancet, 399, 1513–1536, https://doi.org/10.1016/S0140-6736(21)02796-3, 2022. a
Wanner, H., Pfister, C., and Neukom, R.: The variable European Little Ice Age, Quaternary Sci. Rev., 287, 107531, https://doi.org/10.1016/j.quascirev.2022.107531, 2022. a
Wastenson, L., Raab, B., and Vedin, H.: Sveriges nationalatlas: Klimat, sjöar och vattendrag, Stockholm: Sveriges nationalatlas, 176 pp., ISBN 9187760320, 1995. a
Wells, N., Goddard, S., and Hayes, M. J.: A self-calibrating Palmer Drought Severity Index, J. Climate, 17, 2335–2351, https://doi.org/10.1175/1520-0442(2004)017<2335:ASPDSI>2.0.CO;2, 2004. a
Wrigley, E. A. and Schofield, R. S.: The Population History of England 1541–1871, Cambridge University Press, Cambridge, 779 pp., ISBN 9780521356886, 1981. a
Wu, Y., Li, S. S., Zhao, Q., Wen, B., Gasparrini, A., Tong, S. L., Overcenco, A., Urban, A., Schneider, A., Entezari, A., Vicedo-Cabrera, A. M., Zanobetti, A., Analitis, A., Zeka, A., Tobias, A., Nunes, B., Alahmad, B., Armstrong, B., Forsberg, B., Pan, S. C., Iñiguez, C., Ameling, C., Valencia, C. D., Åström, C., Houthuijs, D., Dung, D. V., Royé, D., Indermitte, E., Lavigne, E., Mayvaneh, F., Acquaotta, F., De'Donato, F., Rao, S., Sera, F., Carrasco-Escobar, G., Kan, H. D., Orru, H., Kim, H., Holobaca, I. H., Kysely, J., Madureira, J., Schwartz, J., Jaakkola, J. J. K., Katsouyanni, K., Diaz, M. H., Ragettli, M. S., Hashizume, M., Pascal, M., Cóelho, M. D. Z. S., Ortega, N. V., Ryti, N., Scovronick, N., Michelozzi, P., Correa, P. M., Goodman, P., Saldiva, P. H. N., Abrutzky, R., Osorio, S., Dang, T. N., Colistro, V., Huber, V., Lee, W., Seposo, X., Honda, Y., Guo, Y. L., Bell, M. L., and Guo, Y. M.: Global, regional, and national burden of mortality associated with short-term temperature variability from 2000–19: a three-stage modelling study, Lancet Planet. Health, 6, e410–e421, https://doi.org/10.1016/S2542-5196(22)00073-0, 2022. a