the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Peatland trees record strong and temporally stable hydroclimate information in tree-ring δ13C and δ18O
Kerstin Treydte
Silvia Piccinelli
Loïc Francon
Marçal Argelich Ninot
Johannes Edvardsson
Christophe Corona
Veiko Lehsten
Markus Stoffel
Peatland trees are valuable archives of paleoclimatic information; however, gaps persist in understanding the relationships between tree growth, peatland hydrology, and hydroclimate variables. While previous research in peatlands has mainly focused on tree-ring widths (TRW), yielding inconclusive results, the potential of stable carbon (δ13C) and oxygen (δ18O) isotopes in tree rings remains unexplored. In this study, we develop TRW, δ13C, and δ18O chronologies of Scots pine trees located in a Swedish peatland and a reference site on bedrock with a mineral soil layer. We assess their responses to hydroclimate conditions and evaluate their potential for reconstructing hydroclimate variations. Our findings show significant differences in mean TRW and δ13C values between the peatland and reference sites. Moreover, while TRWs do not exhibit distinct common patterns between sites, both δ13C and δ18O site chronologies show uniform year-to-year variations across all sites. Some discrepancies for TRW and δ13C site chronologies emerge, however, regarding multi-decadal trends. While the climate sensitivity of TRW is weak and non-homogenous, the δ13C and δ18O peatland and reference chronologies contain robust and consistent signals, with a maximum sensitivity to water table, precipitation, and vapor pressure deficit (VPD) variations during summer. Both δ13C and δ18O chronologies show stable relationships with three key hydroclimate variables over time. In conclusion, while TRWs from living peatland pines at our sites have limited potential to record high-frequency hydroclimate information, δ13C and δ18O chronologies can serve as excellent proxies for the reconstruction of past hydroclimate changes.
- Article
(6116 KB) - Full-text XML
-
Supplement
(1396 KB) - BibTeX
- EndNote
Peatlands are significant carbon repositories (Gorham, 1991) and valuable archives of past hydroclimate shifts, as evidenced by various proxies (Chambers and Charman, 2004). Among these, tree rings from both living and subfossil peatland trees can provide unique insights into seasonal climate variability with dating accuracy to the calendar year (Edvardsson et al., 2016). Yet, understanding the complex interactions between climate, tree growth, and peatland hydrology, with the latter being rarely recorded, remains a challenging field of research. Numerous questions persist regarding the main factors influencing tree growth in peatlands (Ballesteros-Cánovas et al., 2022; Dinella et al., 2019; Edvardsson et al., 2015a; Linderholm et al., 2002; Smiljanić et al., 2014; Smiljanić and Wilmking, 2018). In general, tree growth in these environments is linked to the depth and fluctuations of the local water table, making hydrological conditions the main driving factor (Boggie, 1972; Smiljanić et al., 2014). However, rising air temperatures also affect peatland tree growth, both directly by influencing seasonal temperatures and indirectly by enhancing soil evapotranspiration, which reduces waterlogging and ultimately promotes tree growth (Boggie, 1972; Edvardsson et al., 2015b).
Numerous dendroclimatological studies, mostly in Northern Europe, have explored the influence of hydroclimate on peatland tree growth. These studies consistently report challenges with cross-dating, due to missing or wedging rings (Edvardsson et al., 2012b; Pilcher et al., 1995; Smiljanić et al., 2014; Wilmking et al., 2012), and weak, temporally unstable, or non-existent relationships between tree-ring width (TRW) and hydroclimate variables (Cedro and Lamentowicz, 2011; Lamentowicz et al., 2009; Linderholm, 2001; Linderholm et al., 2002). Some studies also evidence complex growth responses that reflect a multiannual synthesis of hydroclimate conditions (Edvardsson et al., 2015a; Edvardsson and Hansson, 2015; Smiljanić et al., 2014). Complex dynamics within peatland ecosystems, including lag and feedback effects, have been suggested as contributing factors to the inconsistent climate-tree growth relationships (Edvardsson and Hansson, 2015; Linderholm et al., 2002; Smiljanić et al., 2014).
To date, systematic dendroclimatological studies utilizing stable isotopes from peatland trees as an alternative to TRW have not been conducted. Unlike traditional tree-ring parameters, such as TRW or maximum latewood density (MXD), stable isotopes are less dependent on specific ecological conditions (Briffa et al., 2002; Saurer et al., 2008; Treydte et al., 2007). This independence allows the use of stable isotopes from various environments, including lowlands, where classical tree-ring parameters often struggle to capture significant climate signals (Cernusak and English, 2015; Hartl-Meier et al., 2015).
Stable carbon (δ13C) and oxygen (δ18O) isotopes in tree rings are valuable proxies reflecting the physiological response of plants to climate and other environmental variables (Gessler et al., 2014; McCarroll and Loader, 2004; Siegwolf et al., 2022). Tree-ring δ13C depends on factors affecting photosynthetic uptake of CO2 and is mainly controlled by stomatal conductance and the rate of carboxylation during photosynthesis (Farquhar et al., 1989; Siegwolf et al., 2022). Such that, warm and dry conditions reduce stomatal conductance and discrimination against 13C, resulting in higher δ13C values (Saurer et al., 1995; Siegwolf et al., 2022).
Tree-ring δ18O reflects the combined influence of the δ18O signature of source water taken up through the roots (Roden et al., 2000), and the leaf water δ18O signature, which is driven by stomatal response to atmospheric vapor pressure deficit (VPD), temperature, and humidity via leaf water 18O enrichment (Barbour et al., 2004). Transpiration regulates the uptake of source water via roots, often sourced from precipitation carrying a specific δ18O signal linked to air mass temperature (Rozanski et al., 1992). The impact of precipitation on δ18O, varies based on changes in the quantity and isotopic composition of infiltrated water (Treydte et al., 2014), soil water evaporation, and groundwater presence (Ehleringer and Dawson, 1992). During warm, dry periods with sufficient soil water, intensified leaf-level evaporative processes can result in increased δ18O values in tree rings (Gessler et al., 2014; Siegwolf et al., 2022).
Several dendroclimatological studies, conducted across central and northern Europe, have consistently reported positive correlations between δ13C and/or δ18O and summer temperature and/or VPD, as well as negative correlations with precipitation and/or moisture. However, these studies also reported variations in the strength and/or temporal stability of responses among sites (Esper et al., 2018; Hartl-Meier et al., 2015; Hilasvuori et al., 2009; Reynolds-Henne et al., 2007; Saurer et al., 1995, 2008; Seftigen et al., 2011; Treydte et al., 2007, 2024), potentially posing challenges for climate reconstructions, particularly when integrating chronologies from various sites, as they may also contain different long-term trends (Esper et al., 2018; Seftigen et al., 2011; Wilmking et al., 2020). These challenges become particularly relevant for hydroclimate reconstructions (Wilmking et al., 2020) based on peatland trees – not only living individuals with known locations, but especially subfossil wood, which may have been relocated from their original growth sites (Eckstein et al., 2009; Edvardsson et al., 2012b, 2014). Moreover, it is plausible that some preserved trees did not grow directly on the peatland surface but instead fell into the margins of developing peatlands, later becoming embedded in the peat (Edvardsson et al., 2016).
Given the research gaps in dendroclimatological studies on peatlands and the potential of stable isotopes as an alternative to TRW, we here developed TRW, δ13C, and δ18O chronologies of Scots pine trees from a Swedish peatland and an adjacent mineral soil site. We assessed the responses of TRW, δ13C, and δ18O to hydroclimate conditions, and evaluated their potential for reconstructing hydroclimate variations. Specifically, we aimed to investigate (i) inter-site differences and similarities in mean values, between chronologies and their multi-decadal trends, (ii) their responses to different hydroclimatic variables such as water table, precipitation, VPD, maximum, minimum, and mean temperatures, and (iii) the temporal stability of the strongest hydroclimatic signals.
2.1 Mycklemossen peatland
Mycklemossen, situated in the Southwestern part of Sweden (58°21′ N 12°10′ E, 80 m a.s.l.; Fig. 1), is a peatland characterized by a mix of wet low areas (hollows) dominated by Sphagnum rubellum and Rhynchospora alba, alongside raised intermediate areas (hummocks) formed by the tussock-building sedge Eriophorum vaginatum. The tussocks comprise drier upper layers of peat, allowing for the establishment of low shrubs such as Calluna vulgaris and vegetation resembling forests with Scots pine (Pinus sylvestris L.) trees (Kelly et al., 2021; White et al., 2023). In general, the thickness of the peat layer across the peatland varies, reaching around 5 m in the center and decreasing to about 1.5 m at the edge. The peatland does not feature a distinct discharge zone, known as a lagg fen, which is typically located at the edge of the peatland and often represents the wettest part of the system (Howie and Meerveld, 2011). Consequently, trees growing at the edge of the peatland neither have their roots submerged in water nor come into significant contact with groundwater throughout the year. The only sources of nutrients and water for the surface vegetation and trees rooted in the acrotelm of the peatland are rainfall and snow.
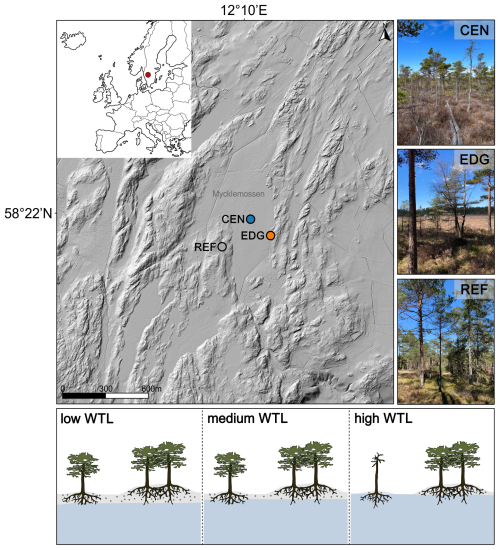
Figure 1Location of the study sites on the Mycklemossen peatland, Sweden (CEN = center, EDG = edge, REF = reference). The red dot on the inset map shows the location of the study area. The figure includes images of the center, edge, and reference sites. Source: © Lantmäteriet (main image with relief shading); own collection (site-specific images). At the bottom, simplified illustrations show trees growing at the Mycklemossen peatland and the hypothetical access of their roots to water pools (blue shading) during low, medium, and high water-table level (WTL). During high WTL, trees growing on a larger hummock (bigger gray-shaded hill) are protected from flooding compared to those on smaller hummocks (smaller gray-shaded hill; own design; not to scale).
The regional climate is characterized by mild winters and cool summers, with an average annual temperature of 6.8 °C. July is the warmest month, with an average temperature of 16.4 °C, while February stands as the coldest (−1.9 °C). Annual precipitation averages 770 mm, peaking in autumn (83 mm in October) and reaching a minimum in winter and spring (44 mm in February–April) according to data from the nearest monitoring station, Vänersborg, covering the 1960–2019 period.
Our sampling sites are located within the Skogaryd Research Catchment, which is included in SITES (Swedish Infrastructure for Ecosystem Science) and ICOS-Sweden (Integrated Carbon Observation System).
2.2 Sampling design
Three sampling sites were established on the Mycklemossen peatland: one in the center (CEN), one at the edge (EDG), and a reference site (REF) (Fig. 1). Although trees at the CEN and EDG sites grow on slightly elevated, drier hummocks, they remain rooted in deep organic soils – up to 5 m in the center and approximately 1.5 m deep at the edge. Despite these micro-elevational differences, both sites are still relatively moist due to the proximity of trees to water pools (Fig. 1). In contrast, the reference site is located on bedrock and features a well-drained mineral soil layer of 10–30 cm thickness, leading to distinctly different hydrological conditions, thus, classified as a dry site. The reference site is assumed to be comparable to other, ecologically non-extreme, temperate sites documented in the literature (Treydte et al., 2007, 2024), where mixed climate signals are recorded in TRW. This comparison allows us to test whether site conditions modulate TRW, δ13C, and δ18O variability and their responses to various hydroclimate variables. In addition to hydrology, the sites also differ in pH and nutrient availability. Trees at the CEN and EDG sites experience lower pH levels and rely solely on nutrients from precipitation. Meanwhile, trees at the REF site benefit from access to both mineral soil nutrients and atmospheric deposition.
Given the challenges associated with cross-dating peatland tree rings, we collected two samples per tree from Scots pine trees at each site (CEN: 20, EDG: 20, REF: 40 trees) using a 5 mm increment borer. The sampled trees showed no visible signs of disturbances such as top-kill, scars, or wood rot. Trees in peatland ecosystems often experience instability due to high water table levels, leading to leaning and the formation of compression wood. Although we observed weak compression wood in some rings of our samples, previous research (Janecka et al., 2020) has shown that this has a minor impact on the climate signal in tree-ring stable isotopes when cellulose is extracted. Consequently, we retained these samples for our analyses.
2.3 Sample preparation and data treatment
First, all cores were cut with a microtome (Gärtner and Nievergelt, 2010), then slightly sanded with a very fine (2000-grit) sanding paper to enhance the visibility of the ring boundaries. The prepared samples were then scanned using a high-resolution scanner (1200 dpi, Epson Expression 10000XL). Tree-ring widths were measured from the resulting high-resolution images using CooRecorder 9.3 (Larsson, 2013). To assign each ring to a calendar year, TRWs were visually crossdated and statistically verified using CDendro (Cybis Electronik & Data, Sweden; Larsson, 2013) and COFECHA (Holmes et al., 1986), respectively. Following cross-dating validation, ensuring that no rings were missing and clearly defined ring borders, five trees per site (one core per tree) were selected for δ13C and δ18O measurements.
For isotope measurements, tree rings from 1960 to 2021 were separated annually using a scalpel under a binocular. Each tree ring was then cut into small pieces and packed into F57 Fiber Bags (200 µm porosity) (ANKOM Technology, Macedon, NY, USA). Cellulose was extracted from the samples using a modified Jayme–Wise Holocellulose Isolation method (Boettger et al., 2007) and was homogenized with an ultrasonic processor UP200S (Hielscher Technology, Teltow, Germany) (Laumer et al., 2009). In total, 915 tree-ring cellulose samples (i.e., 61 tree rings/radius × 15 radii = 915 samples) were individually weighed (1 ± 0.1 mg) and packed into silver capsules for isotope ratio measurements.
The samples were converted to CO by thermal decomposition at 1420 °C with a TC/EA (Pyrocube, Elementar, Hanau, Germany) and C and O isotope ratios simultaneously analysed on an IRMS (MAT 253, Thermo) with a precision of 0.1 ‰. δ13C series were corrected for changes in the atmospheric δ13C value due to anthropogenic activities (Belmecheri and Lavergne, 2020). All subsequent analyses refer to the CO2-corrected data, referred to as “raw δ13C”.
2.4 Hydroclimate data
Gridded monthly climate data (0.5° × 0.5° lat × long) for minimum, maximum and mean temperatures, actual vapor pressure and precipitation sums were obtained from the CRU TS4.06 gridded dataset (Harris et al., 2020). A comparison from the closest gridpoint to our study sites with instrumental records from two nearby meteorological stations (i.e., Kroppefjäll to the north and Såtenäs to the northeast of the study site, both ∼ 30 km away, and the closest CRU grid point ∼ 18 km northwest of the study site) for the overlapping period 1960–2021 revealed highly significant correlations for individual months: minimum temperature (r = 0.90–0.98), maximum temperature (r = 0.76–0.99), mean temperature (r = 0.92–0.98), and precipitation (r = 0.72–0.92). Additionally, stable isotope chronologies were correlated separately with both gridded and instrumental hydroclimate data, revealing similar relationships in each case (analyses conducted but not shown). Given these strong and consistent correlations, we chose to use the CRU dataset for the isotope-hydroclimate analysis in this study.
The atmospheric vapor pressure deficit (VPD) was calculated as the difference between saturated vapor pressure (vp) and actual vapor pressure. While actual vp data were provided by CRU, saturated vp was calculated following Eq. (1) based on Murray (1967):
To estimate the water table levels at Mycklemossen peatland, we utilized half-hourly water table depth measurements from the Skogaryd Research Catchment (SITE project; https://meta.fieldsites.se/resources/stations/Skogaryd, last access: 28 May 2023) that were converted to daily averages covering the period from 20 October 2015 to 31 December 2022. Due to the limited span of this 7-year dataset, we employed the Menyanthes impulse response model (Von Asmuth et al., 2002, 2008) to estimate past water table levels. This statistical model fits daily precipitation and potential evapotranspiration data to water table depth measurements capturing the nonlinear relationship between these daily inputs and water table fluctuations, similar to the approach described by Lehsten et al. (2011). For model input, we used daily minimum, mean, and maximum temperatures and daily precipitation sums from two nearby weather stations (Kroppefjäll-Granan and Såtenäs) maintained by the Swedish Meteorological and Hydrological Institute. Potential evapotranspiration was calculated from minimum and maximum temperatures using the Mcguinnes Bordne formulation, with the implementation of Dey (2024). However, both nearby weather stations experienced either a 10-year downtime or were relocated, making it impractical to rely on a single station. To address this, a δT correction – an adjustment for systematic temperature differences between stations during the overlapping period was applied to harmonize the data from both stations, resulting in a homogeneous weather dataset (r2 > 0.90 for all parameters during the overlapping period). Despite some uncertainties in the station weather data, the model explained 80.6 % of the variance in water table levels over the 7-year period. The root mean square error for predicted water table levels during the measured period was 3.0 cm, indicating reasonable accuracy for the model's estimates.
2.5 Statistical analyses
To analyze the data, we employed several statistical tests. The strength of common variation between the tree-individual isotope time series was tested by calculating the mean inter-series correlation (Rbar) and the expressed population signal (EPS) (Wigley et al., 1984). To account for potential juvenile or non-climatic (e.g., CO2 increase) trends and their typical patterns in the records of the different tree-ring parameters, all individual δ13C and δ18O series were detrended using a 30-year cubic smoothing spline with a 50 % frequency cut-off. TRW series were detrended using a negative exponential curve (Cook and Peters, 1981). While detrending TRW data is essential for climate-growth analysis (Fritts, 1978), there is ongoing debate over the necessity of detrending isotope series (Büntgen et al., 2021; Helama et al., 2015; Torbenson et al., 2022), as the impact on isotope-climate responses can vary significantly (Esper et al., 2018). Consequently, we used both non-detrended (“raw”) and spline-detrended (“detrended”) stable isotope time series for correlations with hydroclimate data focusing on the most robust agreements. In total, we developed 15 chronologies (3 × TRW, 3 × δ13C raw, 3 × δ13C detrended, 3 × δ18O raw, 3 × δ18O detrended) by averaging the corresponding individual time series using the biweight robust mean (dplR package in R; Bunn et al., 2012). Descriptive statistics were calculated for all raw and detrended TRW, δ13C, and δ18O series over the common 1960–2021 period.
Our raw TRW data deviated from a normal distribution, whereas the isotope data conformed to a normal distribution as determined by the Shapiro–Wilk test – a characteristic often observed in isotope data (Treydte et al., 2024). Consequently, we used the Wilcoxon test for raw TRW and t test for raw δ13C and δ18O data to compare mean values across the three study sites. Additionally, we used Pearson's correlation coefficient to quantify the relationships between site-specific detrended TRW and raw δ13C and δ18O chronologies including their low frequency components. Statistical comparisons between tree-ring parameters were conducted by calculating Pearson's correlation coefficients between detrended TRW and raw δ13C and δ18O chronologies, as well as their multi-decadal trends.
To investigate the relationships between TRW, δ13C and δ18O variations and hydroclimate conditions, we calculated bootstrapped Pearson's correlation coefficients between the six raw (δ13C and δ18O) and nine detrended (TRW, δ13C and δ18O) site chronologies and monthly hydroclimate variables (water table, precipitation and VPD, but also maximum, minimum, and mean temperatures) over the 61-year period from 1960 to 2021. The significance of the correlation coefficients was tested using a bootstrapping procedure with 1000 iterations (treeclim package in R; Zang and Biondi, 2015). This analysis spanned 21 individual months, from March of the year before xylem cell formation to October of the current year as well as the June–August season (δ13C and δ18O only). Statistical significance was determined at p < 0.05.
Building on the static correlation outcomes, we identified the season with the most robust link between δ13C, δ18O and hydroclimate conditions. To examine the stability of these relationships over time, we applied a bootstrapped correlation analysis using 31-year moving windows lagged by 1 year, over the 1960–2021 period with 1000 iterations.
3.1 Chronology characteristics
The oldest trees were found at REF, where the average tree age was 118 years, with a maximum age of 142 years and a minimum age of 92 years. Trees at CEN and EDG were of similar age, with average values of 77 and 83 years, respectively. The oldest and youngest trees at CEN were 127 and 53 years old, while those at EDG were 116 and 44 years old.
For TRW weak to strong common variations were found among tree-individual raw (Fig. 2) and detrended time series, with low to very high values of Rbar and EPS (Table 1). The lag-1 autocorrelation values (AR1; Table 1) were very high for the raw TRW chronologies and decreased after detrending.
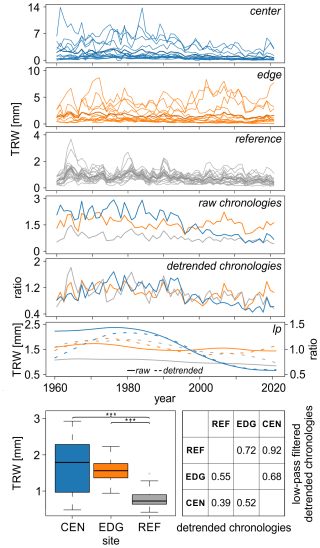
Figure 2Tree-ring width (TRW) data spanning from 1960 to 2021 displayed for the center (CEN), edge (EDG), and reference (REF) sites; the raw and detrended chronologies; and the 30-year low-pass filters for both the raw (solid lines) and detrended (dashed lines) chronologies. The boxplots show the differences in mean raw TRW values across the three sites ( < 0.001). The table presents the correlations between detrended TRW site chronologies and 30-year low pass filters of the detrended TRW site chronologies. Please, note, that the y-axis scales differ between panels presenting individual series.
Table 1Characteristics of the raw and detrended TRW, δ13C, and δ18O data from the center (CEN), edge (EDG), and reference (REF) sites. Rbar = mean inter-series correlation; EPS = expressed population signal; AR1 = first-order autocorrelation.
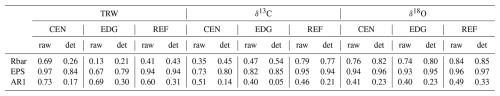
For δ13C and δ18O strong common variations were found between the tree-individual raw time series at all sites (Fig. 3a and b), with moderate to very high values of Rbar and EPS (Table 1). Overall, Rbar and EPS values were slightly higher for detrended than raw data. Rbar values were higher for δ18O than for δ13C, similarly to EPS values (Table 1). Lag-1 autocorrelation values of the raw isotope chronologies were similar for both δ13C and δ18O. After detrending, the Lag-1 autocorrelation across all stable isotope chronologies decreased (Table 1).
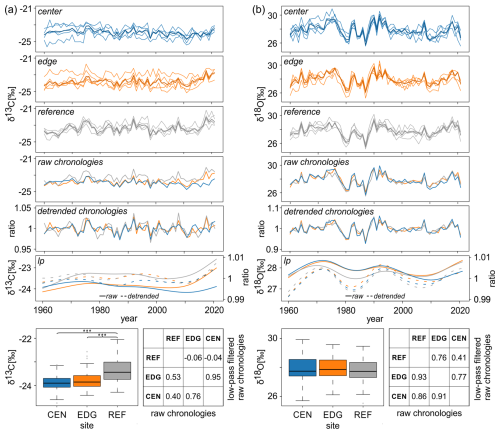
Figure 3Tree-ring (a) δ13C and (b) δ18O data spanning from 1960 to 2021. Each panel (a) and (b) presents the raw series from the center (CEN), edge (EDG), and reference (REF) sites; the corresponding raw and detrended chronologies; and 30-year low-pass filters of the raw (solid lines) and detrended (dashed lines) chronologies. Boxplots show the differences in mean raw δ13C and δ18O values across all three sites ( < 0.001). Tables display correlations between raw δ13C and δ18O site chronologies and 30-year low pass filters of the raw chronologies.
3.2 Comparison of site chronologies
The mean raw TRW values at the REF site were the lowest and significantly differed from those at the CEN and EDG sites of the Mycklemossen peatland (p < 0.001; see boxplots in Fig. 2). The highest values were recorded at CEN (1.68 mm). Pearson correlation analysis of the detrended TRW chronologies across the sites indicated weak to medium coherence, with r values ranging from 0.39 (p < 0.01) to 0.55 (p < 0.001); see table in Fig. 2).
The low-pass filtered detrended TRW site chronologies (i.e., 30-year splines) revealed some discrepancies, including a distinct negative trend at CEN, that was not present at EDG and REF (Fig. 2). The correlations between the site chronologies ranged from 0.68 to 0.92 (p < 0.001) (see table in Fig. 2).
Mean raw δ13C values at REF significantly differed from the two peatland sites CEN and EDG (p < 0.001; see boxplots in Fig. 3a). The highest δ13C values were found at REF (−23.36 ‰), with the lowest recorded at CEN (−23.91 ‰). However, the mean raw δ13C values of CEN and EDG did not differ significantly (see boxplots in Fig. 3a). In contrast, the mean δ18O values across all three sites, ranging from 27.80 ‰ at REF to 27.95 ‰ at EDG did not show statistically significant differences (see boxplots in Fig. 3b).
Comparison of the raw isotope chronologies showed strong coherence between sites. Pearson correlation analysis indicated highly significant correlations between sites for both δ13C and δ18O, with even stronger correlations for δ18O (r values between 0.86 and 0.93) compared to δ13C (r values between 0.40 and 0.76) (p < 0.001; see table in Fig. 3b and a).
Low-pass filtered raw site chronologies (i.e., 30-year splines) showed some discrepancies with increasing trends in δ13C (Fig. 3a) and δ18O (Fig. 3b) values over the most recent 15 years and more decadal variation in δ18O (Fig. 3b) compared to δ13C (Fig. 3a). The correlations between sites for δ13C ranged from −0.06 (non-significant) to 0.95 (p < 0.001) (see table in Fig. 3a) while for δ18O they ranged from 0.41 to 0.77 (p < 0.001) (see table in Fig. 3b).
3.3 Relationships between TRW, δ13C, and δ18O chronologies and their multi-decadal trends
Comparisons between the TRW chronologies detrended using a negative exponential function and raw δ13C and δ18O chronologies indicated varying degrees of correlation, ranging from non-significant to highly significant (p < 0.001) relationships (Fig. 4a). The correlations between the detrended TRW and raw δ13C chronologies ranged from −0.34 (p < 0.01) to 0.46 (p < 0.001). Similar patterns were observed between the detrended TRW and raw δ18O chronologies, with values ranging from −0.04 (non-significant) to 0.45 (p < 0.001) (Fig. 4a).
The correlations between the low-frequency trends in detrended TRW and raw δ13C and δ18O chronologies varied from weak to strong (Fig. 4b). The values ranged from −0.40 (p < 0.05) to 0.79 (p < 0.001) for TRW vs. δ13C and from −0.21 (non-significant) to 0.79 (p < 0.001) for TRW vs. δ18O (Fig. 4b). Generally, the relationships between tree-ring parameters at the REF site were weak or non-significant, while at the CEN site, all correlations were significant.
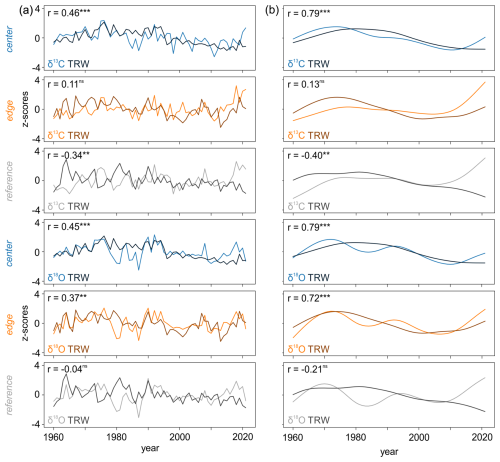
Figure 4The relationship between (a) detrended TRW and raw δ13C and δ18O chronologies and (b) 30-year low pass filters of detrended TRW and raw isotope chronologies ( < 0.001, < 0.01, *p < 0.05, ns = non-significant).
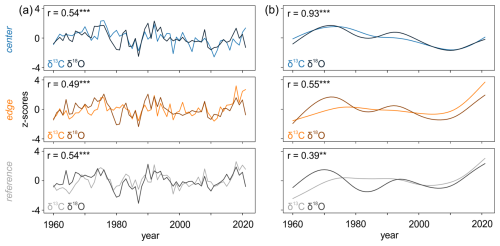
Comparisons of the raw δ13C and δ18O chronologies indicated strong and significant (p < 0.001) relationships (Fig. 5a), with correlations ranging from 0.49 to 0.54. The low-frequency trends of the raw δ13C and δ18O chronologies were strongly correlated at CEN with r = 0.93 (p < 0.001). However, differences were more marked at the REF site, where the correlation was lower (r = 0.39, p < 0.01) (Fig. 5b). In general, the low-frequency domain of the raw δ18O chronologies showed more pronounced decadal variations compared to the δ13C chronologies.
3.4 Relationship of the TRW, δ13C, and δ18O chronologies to hydroclimate conditions
The relationships between detrended TRW site chronologies and hydroclimate variables were generally weak, with no consistent response patterns emerging across the sites (Fig. 6). The most significant correlations were found for June of the year preceding xylem cell formation. Specifically, negative correlations were found between TRW and water table (with a maximum significant r value of −0.38 at EDG) and precipitation (maximum significant r value = −0.40 at CEN). Conversely, positive correlations were observed between TRW and VPD (maximum significant r value = 0.42 at EDG) and temperatures (maximum significant r value of 0.36 for maximum temperature at EDG).
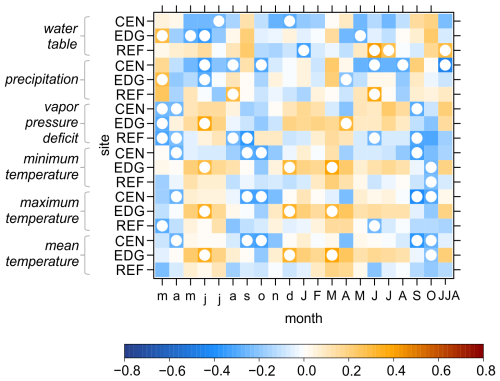
Figure 6Pearson correlation coefficients between the detrended tree-ring width site chronologies (CEN = center, EDG = edge, REF = reference) and hydroclimate variables. These variables include raw instrumental (water table) and raw gridded (precipitation, vapor pressure deficit, minimum, maximum, and mean temperatures) data. The correlations were computed for the period from March of the year preceding xylem cell formation to October of the current year and combined June–August (JJA). White circles indicate significant correlation at p < 0.05.
Between all δ13C and δ18O site chronologies and hydroclimate variables strong and consistent relationships were observed. Both raw and detrended isotope chronologies showed nearly identical climate signals (Fig. 7: raw; Fig. S1 in the Supplement: detrended) and hence, only the results from the raw chronologies are presented here. Particularly strong correlations were found with variations of the water table, precipitation (negative correlations) and VPD (positive correlations) during the summer months (June to August) of the year in which the ring was formed (Figs. 7 and S2). Although all correlations were highly significant, (p < 0.05), r values were slightly higher for the δ13C chronologies compared to the δ18O chronologies. Correlations with minimum, maximum, and mean temperatures were slightly weaker in comparison to those with hydroclimatic variables. In addition, the seasonal response differed between δ13C and δ18O: for the δ13C chronologies, the strongest correlation remained most apparent during the summer months (June to August) particularly with maximum temperature, whereas for δ18O, significant relationships shifted to the winter/early spring months (January to March) (Fig. 7).
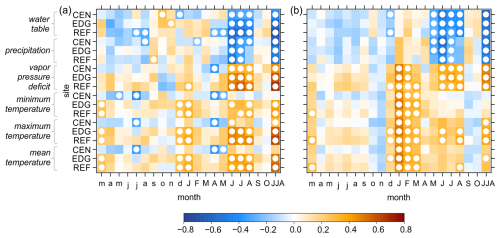
Figure 7Pearson correlation coefficients between the raw (a) δ13C and (b) δ18O site chronologies (CEN = center, EDG = edge, REF = reference) and hydroclimate variables. These variables include raw instrumental (water table) and raw gridded (precipitation, vapor pressure deficit, minimum, maximum, and mean temperatures) data. The correlations were computed for the period from March of the year preceding xylem cell formation to October of the current year and combined June–August (JJA). White circles indicate significant correlation at p < 0.05.
3.5 Main hydroclimate drivers of δ13C and δ18O variations
Overall, the primary drivers of δ13C and δ18O variations across all three sites were the water table, precipitation, and VPD during the summer months (June to August) (Figs. 7 and 8). While the strength of the correlations varied between isotopes and sites, δ13C generally showed slightly higher correlations than δ18O, although the differences were not consistent. Specifically, δ13C showed increasing correlations from CEN to REF for water table and VPD, with the strongest precipitation signal found at CEN (Fig. 8a). Interestingly, although δ18O presented comparable correlations across sites, the two sites located within the peatland, i.e., CEN and EDG exhibited even stronger responses (p < 0.001) to all three hydroclimate variables compared to the REF site (Fig. 8b). The correlation values ranged from moderate to high for δ13C (Fig. 8a) and δ18O (Fig. 8b) chronologies.
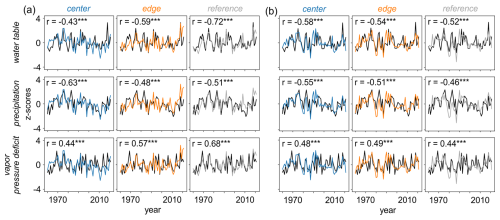
Figure 8Relationship between raw (a) δ13C and (b) δ18O site chronologies and June–August instrumental (water table) and gridded (precipitation and vapor pressure deficit) hydroclimate data ( < 0.001). Water table and precipitation data are presented with reversed y axes for better clarity in interpreting the correlations.
3.6 Temporal robustness of the summer hydroclimatic signals in δ13C and δ18O
Summer (JJA) of the current year of tree-ring formation was identified as the season with the highest correlations between the isotope chronologies and hydroclimate variables. To assess the temporal robustness of these signals, this period was selected for further analysis. Across all three sites significant relationships were consistently observed between δ13C (Fig. 9a) and δ18O (Fig. 9b) and water table, precipitation amount and VPD throughout the entire analysis period.
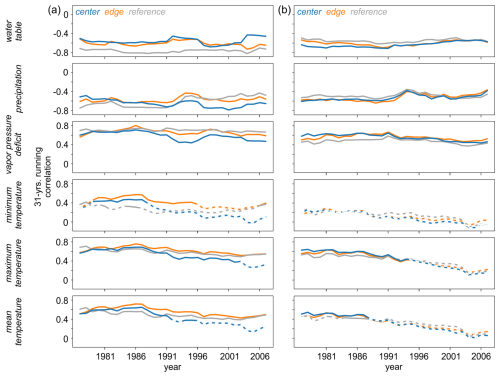
Figure 9Temporal stability of the raw (a) δ13C and (b) δ18O site chronologies and hydroclimate data (water table, precipitation, vapor pressure deficit, minimum, maximum, and mean temperatures), calculated using a 31-year moving correlation. Solid lines indicate statistically significant correlations at p < 0.05.
In addition, δ13C showed significant correlations with maximum and mean temperatures over time at all three sites, although there was a brief loss of sensitivity at the CEN site in recent years. The least stable and weakest, or even non-significant relationships were found between both isotopes and minimum temperature, as well as between δ18O and maximum and mean temperatures. Results slightly deviated for the detrended chronologies (Fig. S3) with minimum temperature becoming more significant for δ13C, whereas the water table and precipitation signals diminished in recent years.
Our research offers a novel perspective on the complex interactions between climate, peatland hydrology, and tree growth. By examining not only tree-ring width, but also δ13C and δ18O chronologies of Scots pine in Southwestern Sweden, we provide new insights into peatland dendroclimatology. A key finding of our study is that the same hydroclimate variables influence both and fixation processes in tree rings. Furthermore, we find that while these variables significantly impact the isotopic signatures, local site conditions – such as the distinction between peatland and bedrock environments with a mineral soil layer – subtly affect δ13C and δ18O variability and their relationships with hydroclimatic conditions. Consistent with previous studies on peatland pines, our findings show that the relationships between TRW and hydroclimate conditions are weak and inconsistent, making it difficult to pinpoint specific factors limiting tree growth.
4.1 Differences and similarities between site chronologies
4.1.1 Tree-ring width
In peatlands, tree growth is particularly sensitive to fluctuations in the water table, which influences root access to both water and nutrients and reduces soil aeration. Elevated water tables limit oxygen and nutrient availability, leading to environmental stress that typically manifests as narrow tree rings, while lower water tables tend to promote radial growth (Boggie, 1972; Penttilä, 1991; Freléchoux et al., 2000; Vitas and Erlickytë, 2007). Additionally, shallow rooting and unstable ground conditions – common in peatland ecosystems – can cause trees to tilt, triggering the formation of compression wood and eccentric growth as mechanical response to ground instability (Malinen et al., 2005; Timell, 1986). Wind and repeated ground movement may additionally alter the direction of compression wood within a tree, further distorting growth patterns (Linderholm et al., 2002; Zoltai and Pettapiece, 1974). As a result, tree rings may alternate between extremely narrow and unusually wide – varying individually among trees – reflecting the combined effects of environmental and mechanical stress. Such non-climatic influences can mask or distort the regional climate signals recorded in tree growth patterns. In contrast, mineral soil developed over bedrock provides markedly different growing conditions: it is well-drained, offers moderate to high nutrient availability, and ensures stable rooting conditions (He et al., 2025). While these characteristics may generally support more uniform growth, water availability can still be a limiting factor. The underlying granitic bedrock, although capable of storing substantial water volumes for trees (Nardini et al., 2024) due to its high porosity from weathering processes (Migoń and Lidmar-Bergström, 2001), does not necessarily provide readily accessible moisture for tree roots. This restricted access to water, despite favourable soil properties, may contribute to lower overall growth. However, due to the more homogeneous environmental conditions compared to the peatland, tree growth on mineral soil tends to be more synchronized (Linderholm et al., 2002).
Trees on the Mycklemossen peatland, similar to those in other European peatlands (Becker et al., 2008; Edvardsson et al., 2016; Holmgren et al., 2015; Smiljanić and Wilmking, 2018), grow on slightly elevated, drier hummocks (Fig. 1), which offer some protection from excessive flooding, while maintaining water access, typically fluctuating between 0 and −30 cm in June–August (based on modelled data from 1960 to 2021). Therefore, as mentioned before, the presence of these hummocks and/or a lower water table likely contributes to wider TRW compared to the reference site, which offers comparatively drier growing conditions. However, the relatively non-synchronous growth observed between peatland sites is likely driven by at least four interacting factors: (i) individualistic responses of trees to drier conditions on hummocks (Carrer, 2011), (ii) variation in hummock height, leading to micro-environmental differences in growth conditions (Pouliot et al., 2011), (iii) the shallower peat at the edge (∼ 1.5 m), potentially forming a transitional zone between the central peatland (with up to 5 m of peat) and the adjacent mineral soil, and (iv) localized shifts in hydrology, unstable ground conditions, and wind exposure, leading to episodes of compression wood formation, eccentric growth, and growth suppression followed by release (Linderholm et al., 2002; Malinen et al., 2005; Timell, 1986; Zoltai and Pettapiece, 1974). In addition, differences in growth patterns between the peatland and the reference site likely reflect the contrasting soil conditions: the thin mineral soil layer at the reference site (10–30 cm) offers markedly different growing conditions than the deep organic soils of the peatland (He et al., 2025). The weak to moderate coherency in growth patterns observed in our study (Fig. 2) is consistent with previous research comparing Scots pine TRWs on peatlands and mineral soils (Cedro and Lamentowicz, 2011; Hökkä et al., 2012; Linderholm et al., 2002). In comparison, a notable example comes from a study in Lithuania, where TRW chronologies from pine populations growing just a few hundred meters apart – on peatland and mineral soils – showed no significant correlation (Edvardsson et al., 2015b). In this context, the weak to moderate relationships observed in our study suggest that, while TRWs do share a common signal, it is still limited. This reinforces our assumption that tree growth across our sites is influenced by multiple co-existing environmental and site-specific factors (Edvardsson et al., 2015a, 2016; Linderholm et al., 2002). As a result of this complexity, developing chronologies that are coherent across ecologically distinct sites – or even among different locations within the same peatland – can be challenging (Smiljanić et al., 2014).
The moderate relationships in the multi-decadal trends of the three TRW chronologies (Fig. 2) align with findings from Lithuania (Edvardsson et al., 2015a) indicating that once peat soil becomes saturated with water, short-term changes in temperature or precipitation may not significantly alter the hydrological conditions that affect tree growth. Some studies (e.g., Gore and Goodall, 1983; Charman et al., 2004) suggest that hydrological responses in peatlands tend to be slow, driven primarily by persistent climate patterns rather than short-term fluctuations.
4.1.2 δ13C and δ18O
Significant difference in mean δ13C values among the sites, with the lowest values recorded at the peatland sites (Fig. 3) likely arise from variations in local site conditions. The peatland sites, although located on drier hummocks, still provide trees with access to water pools (Fig. 1). In contrast, the reference site, located on bedrock, has a shallow mineral soil layer with higher water permeability. As mentioned before, although the granitic bedrock can retain substantial water for trees (Nardini et al., 2024), the growing conditions vary significantly between the peatland and reference sites, leading to noticeable differences in mean δ13C values. The greater water availability at the peatland sites allows for higher stomatal conductance, resulting in lower δ13C values compared to the reference site (Saurer et al., 1995; Siegwolf et al., 2022). One might expect that the generally higher water availability in peatlands would reduce stomatal aperture due to potentially anaerobic conditions (Kozlowski, 1997, 1984) resulting in lower discrimination against 13C and higher δ13C values (Saurer et al., 1995; Siegwolf et al., 2022). Trees in the Mycklemossen peatland, however, grow on hummocks, with convenient yet not excessive access to water pools (Fig. 1) and shallow, horizontally extended root systems (> −20 cm, Heikurainen, 1955; He et al., 2023). Therefore, stomatal conductance of these trees may remain high, leading to lower δ13C values. In contrast, higher δ13C values (Fig. 3) observed alongside narrower tree rings (Fig. 2) at the reference site underscore its dependence on soil moisture and indicate drought stress (Leavitt, 1993; Saurer et al., 1995; Treydte et al., 2014). The highest δ13C values recorded at this driest site suggest reduced stomatal conductance and increased water-use efficiency (Gessler et al., 2014; Saurer et al., 1995, 2014; Treydte et al., 2001).
The proximity of our sites excludes differences in the influence of precipitation δ18O signatures and vapor pressure deficit on mean tree-ring δ18O values, which exhibit remarkable similarity, as observed by Hartl-Meier et al. (2015) and Esper et al. (2018) at locally diverse (moist vs. dry) sites. Despite the ecological differences between our peatland and bedrock sites, the root systems at all locations are limited to the upper 0–30 cm of the soil layers, where only rainfall, including snow in winter, serves as the sole water source. It is widely acknowledged that soil water close to the surface is evaporatively enriched in 18O compared with deeper soil water pools (Sarris et al., 2013; Treydte et al., 2014). This would suggest that trees at each location rely on 18O enriched surface water, resulting in comparable tree-ring δ18O values.
Although we documented strong similarities in the mean values and site-specific raw chronologies, particularly for δ18O (Fig. 3b), multi-decadal trends slightly differ, especially for δ13C (Fig. 3a). These discrepancies in δ13C multi-decadal trends, likely result from the complex interaction between climate dynamics and site-specific conditions. Specifically, the increase in air temperature (Fig. S4) due to climate change (Joelsson et al., 2023) likely enhances soil evapotranspiration differently across these ecologically diverse sites, creating slightly varied growth conditions (Li et al., 2014). The more positive multi-decadal trends in δ13C at the edge and reference sites over the past decade suggests that rising air temperature (Fig. S4) may have intensified soil water transpiration more at these sites compared to the center, leading to drier conditions, narrower stomata, and higher δ13C values, particularly at the reference site. Conversely, the generally high-water levels at the center site may have buffered against the effects of rising air temperatures (Fig. S4) and intensive soil evapotranspiration leading to lower δ13C values. The difference in multi-decadal trends between peatland sites highlights the importance of careful trend examination in isotope data and avoiding the mixing of trees from different sites in climate reconstruction studies, as this could introduce artifacts that are difficult to statistically estimate (Esper et al., 2018).
In summary, the consistent isotope signals observed across our three study sites align with findings from numerous studies conducted outside peatland environments (e.g., Hartl-Meier et al., 2015; Klesse et al., 2018; Saurer et al., 2008; Treydte et al., 2007, 2024). These studies have demonstrated that stable isotope values in tree rings are generally less affected by local site conditions than traditional tree-ring parameters such as TRW. This relative insensitivity enhances their value for dendroclimatological research, particularly in environmentally heterogeneous regions like lowlands, where TRW often lacks coherence and fails to reliably reflect climatic variability (Cernusak and English, 2015; Hartl-Meier et al., 2015).
4.2 Relationships between TRW, δ13C, and δ18O data
We analysed the statistical relationships between detrended TRW and raw δ13C and δ18O chronologies (Fig. 4a) as well as the multi-decadal trends extracted from these chronologies (Fig. 4b). Notably, we found strong positive correlations between these chronologies, particularly at the central peatland site, where both TRW vs. δ13C and TRW vs. δ18O relationships were highly significant. The positive relationship between detrended TRW and raw δ13C chronologies at the central peatland site contrasts with the finding of Hartl-Meier et al. (2015), who reported a negative correlation, suggesting that dry conditions reduce carbon fixation and, consequently, suppress tree growth. Interestingly, our findings also indicate that a drop in water level reduces carbon fixation, resulting in higher δ13C values (Fig. 3a). However this reduction in water level simultaneously promotes tree growth, a phenomenon typically observed when higher water table levels suppress growth (e.g., Edvardsson et al., 2016; Smiljanić and Wilmking, 2018). This suggests that under drier conditions, both in short- and longer-term contexts, trees may benefit from increased nutrient availability in the soil (Freléchoux, et al., 2000; Penttilä, 1991). Additionally, as proposed by Edvardsson et al. (2014), trees may also utilize stored carbohydrates from previous years to produce wider rings further explaining the positive relationship between TRW and δ13C under these conditions.
The observed positive relationship between detrended TRW and raw δ18O chronologies at the central peatland site initially suggests that slightly lowered water tables and relatively drier surface conditions on hummocks may promote tree growth (Edvardsson et al., 2016; Smiljanić and Wilmking, 2018). Under such conditions, trees may benefit from improved soil aeration while still accessing sufficient moisture, enabling continued stomatal conductance and carbon uptake even under atmospherically dry conditions (i.e., high VPD). Importantly, higher δ18O values do not necessarily indicate soil water limitation or drought stress, but rather enhanced leaf-level evaporative enrichment driven by high atmospheric water demand. When soil moisture is adequate, stomatal conductance will remain high also under high VPD, enabling both continued transpiration and xylem cell production. Therefore, the observed positive relationship between δ18O and TRW likely reflects conditions where trees experience high evaporative demand but no major constraints on water uptake, supporting both isotopic enrichment and radial growth. The strength of the relationships between TRW, δ13C, and δ18O, however, varies across sites (Figs. 4 and 5), ranging from strong to weak or non-significant. This underscores the complex interplay between local hydrology and atmospheric conditions and highlights that wood production in peatland pines cannot be inferred from stomatal behaviour alone, but must be understood within site-specific environmental contexts.
4.3 Relationships between δ13C and δ18O records
We evaluated the strength of the relationships between raw δ13C and δ18O chronologies (Fig. 5a) and their multi-decadal trends (Fig. 5b). Strong correlations between the δ13C and δ18O chronologies are consistent with findings from an isotope network across Europe (Treydte et al., 2007) supporting our hypothesis that these isotopic signatures are predominantly linked through leaf-level processes in the high-frequency domain (Treydte et al., 2007).
Interestingly, the relationships between multi-decadal trends show significant variability (Fig. 5b). The peatland site located at the center show a notably high correlation while the edge and reference sites exhibit moderate to low correlations, respectively. Generally, the low correlations between multi-decadal δ13C and δ18O trends can be attributed to at least two factors. First, δ13C records are influenced by tree size and stand dynamics, whereas δ18O records are less affected by these factors (Klesse et al., 2018). Second, during the industrial period (20th/21st century), multi-decadal trends in δ13C records may reflect non-climatic influences such as changes in stomatal conductance in response to rising atmospheric CO2 concentrations (Treydte et al., 2009). In contrast, δ18O records tend to have minimal to no non-climatic trends (Young et al., 2011), preserving longer-term climatic variation more reliably (Treydte et al., 2024). While combining δ13C and δ18O can enhance climate signal (Loader et al., 2008; Treydte et al., 2007) and previous (hydro)climate reconstructions have combined both δ13C and δ18O data (Bégin et al., 2015; Büntgen et al., 2021), it is imperative to individually examine the long-term trends of each isotope chronology before merging the data. This careful examination helps prevent the conflation of divergent long-term trends and ensures the integrity of climate reconstructions (Treydte et al., 2007).
4.4 Hydroclimate signals
4.4.1 Tree-ring width
Our findings reveal weak and non-systematic (Fig. 6) relationships between detrended TRW chronologies and hydroclimate variables, a result consistent with other studies (e.g., Linderholm, 2001; Linderholm et al., 2002; Lamentowicz et al., 2009; Cedro and Lamentowicz, 2011; Edvardsson et al., 2015a; Edvardsson and Hansson, 2015). Significant responses were mostly observed in June of the year preceding xylem cell formation and were evidenced only at the peatland sites. At the reference site, however, TRWs showed a positive correlation with precipitation in both the year preceding and the year of xylem formation (Fig. 6), indicating that trees growing on mineral soil experience drought stress. In general, the limited and delayed responses are likely due to the reliance of peatland pines on fluctuations in the water table, in addition to direct effects of climate on growth (Linderholm et al., 2002).
Climate changes may not immediately affect the water table level which can take a few years to several decades to respond (Kilian et al., 1995). Our findings regarding the influence of past climate on peatland pine growth support the hypothesis of this lag (Edvardsson and Hansson, 2015; Linderholm et al., 2002; Smiljanić et al., 2014) and the idea that TRWs in peatland pines may not be suitable for high frequency hydroclimate reconstructions (Linderholm et al., 2002).
4.4.2 δ13C and δ18O
We observed strong inter-site similarity between δ13C and particularly δ18O chronologies (Fig. 3) suggesting a common hydroclimate signal (Saurer et al., 2008). This is confirmed by our dendroecological analysis (Fig. 7), which shows that summer hydroclimate conditions, particularly water table, precipitation, and vapor pressure deficit (Fig. 8), are key drivers of δ13C and δ18O variations. These results are consistent with findings documented at ecologically diverse sites in central and northern Europe (Esper et al., 2018; Hartl-Meier et al., 2015; Saurer et al., 2008; Treydte et al., 2024). The minor relevance of previous-year conditions also align with other studies (Esper et al., 2018; Hartl-Meier et al., 2015; Reynolds-Henne et al., 2007; Seftigen et al., 2011; Treydte et al., 2024), indicating that carryover effects into subsequent tree-ring cellulose δ13C and δ18O are negligible. This suggests that if water transport is sufficient (Martínez-Sancho et al., 2023), the isotopic signature of tree-ring cellulose is predominantly created during the peak of meristematic activity and xylem cell-wall thickening in summer (Cuny et al., 2015; Treydte et al., 2024). This assumption is also confirmed by the dendrometer and wood phenological investigation performed on the Mycklemossen peatland, in parallel to this isotope study (Francon et al., 2024). The positive correlations between TRW and δ13C, suggesting that trees from the central peatland utilize stored carbohydrates to produce wider rings, may therefore be disregarded. Subtle yet consistent differences in the δ18O response strength reveal that peatland sites exhibit higher sensitivity to key summer hydroclimate parameters compared to the reference site on bedrock (Fig. 8b). This suggests that δ18O chronologies from peatland sites are more reliable proxy for past hydroclimate changes. Higher soil water availability in peatland likely facilitates stomatal opening and continued transpiration even under high VPD conditions resulting in evaporative leaf water 18O enrichment and higher isotopic values in the newly produced assimilates utilized for cellulose synthesis (Gessler et al., 2013, 2014; Offermann et al., 2011; Treydte et al., 2014). The weaker correlations at the reference site suggest less sensitivity to climate variables potentially due to more frequent stomatal closure and less variation in δ18O in response to climate. This implies that under moist peatland conditions, the relative variations in leaf water enrichment due to increased transpiration outweigh the relative variations in soil water δ18O, leading to stronger associations between hydroclimate and δ18O at these locations (Hartl-Meier et al., 2015). Despite similar mean values across sites (Fig. 3), the stronger correlations between δ18O and hydroclimate parameters at peatland sites and positive correlations between TRW and δ18O (Fig. 4), suggest that soil moisture content and stomatal size are not the sole drivers of δ18O variations. Simultaneously, we cannot exclude that trees at all sites solely utilize surface water enriched in 18O. These findings are consistent with those reported by Treydte et al. (2014), Hartl-Meier et al. (2015), and Esper et al. (2018), indicating that climate signals in tree-ring δ18O variations are most pronounced at temperate sites with humid conditions.
Additionally, we observed a positive impact of winter/early spring (January to March) temperatures on δ18O (Fig. 7). This relationship could be attributed to snowmelt dynamics. Warmer temperatures can induce snowmelt or rainfall leading to isotopic enrichment in surface water due to evaporation and sublimation processes (Dahlke and Lyon, 2013). This isotopically enriched water can be incorporated into tree rings, resulting in higher δ18O values. Warmer temperatures also accelerate snowmelt, potentially reducing snowmelt water availability and increasing reliance on rainwater. Trees in peatlands and at the reference site, constrained by shallow root systems or bedrock (He et al., 2023; Heikurainen, 1955), may thus use isotopically enriched surface water, contributing to higher δ18O values.
A distinct pattern, similar to the one observed for δ18O, is not evident in the δ13C responses to hydroclimate parameters. While mean δ13C values are consistently lower at the peatland sites compared to the reference site (Fig. 3), the strength of responses to key hydroclimate parameters varies (Fig. 8). This suggests that the hydroclimate signal preserved in δ13C is more susceptible to influences from differences in site conditions, resulting in greater variability in response strength. δ13C appears to be a more sensitive indicator of environmental stress compared to δ18O, as variations in stomatal conductance are more pronounced at the reference site than at the peatland sites. Trees at the reference site must regulate transpiration more carefully to prevent excessive water loss which supports our previous finding that stomata tend to remain more closed at the reference site. This results in higher δ13C values (Fig. 3) and stronger relationships with the water table (Fig. 8).
We also observed stronger δ13C responses to VPD at the reference site, suggesting that trees adjust their carbon assimilation processes in response to the combined effects of soil and atmospheric conditions (Fonti et al., 2013; Zeiger and Farquhar, 1987). Specifically, reduced soil moisture and higher VPD may induce stomatal closure in trees as a response to water stress. This, in turn, affects carbon assimilation, leading to higher δ13C values in tree rings and a stronger correlation between δ13C and VPD (Saurer et al., 2004).
In addition to the strong, stationary hydroclimate-isotope relationships (Figs. 7 and 8), we observed significant correlations between key hydroclimate parameters and both isotopic parameters over time (Fig. 9). These findings of temporally stable and significant responses align with previous studies conducted in central and northern Europe (Reynolds-Henne et al., 2007; Treydte et al., 2024). Our results indicate that despite variations in climate and site conditions, three key hydroclimate parameters – likely including water table level, precipitation, and vapor pressure deficit – remain consistently important for our δ13C and δ18O data throughout the analysed period. Contrasting findings were reported for sites in the Central Scandinavian mountains (Seftigen et al., 2011), where temporally unstable precipitation and/or temperature–δ18O and δ13C relationships were observed. These unstable relationships were associated with large-scale shifts in climate and changes in the regional precipitation pattern. The temporal stability observed in our study is particularly noteworthy because the consistency of the hydroclimate signal registered in tree rings is crucial for potential hydroclimate reconstructions (Wilmking et al., 2020; Treydte et al., 2024). Any deviation from the assumption of temporal stability could lead to erroneous estimations of past temperature, precipitation, or water table trends, extremes, amplitudes, or drought severities in tree-ring-based reconstructions (Wilmking et al., 2020).
Our study is the first to examine Scots pine TRW, δ13C and δ18O patterns and their responses to hydroclimate conditions in a peatland environment in Southwestern Sweden. Our findings reveal substantial differences in TRWs mean values across sites with weak to moderate relationships between site-specific chronologies. However, relatively strong relationships in multi-decadal trends are observed. Notable discrepancies also exist in both mean values and multi-decadal trends of δ13C. In contrast, mean δ18O values, site-specific chronologies, and multi-decadal trends exhibit strong and significant similarities across all sites.
Both δ13C and δ18O records from peatland trees show robust responses to summer hydroclimate conditions, mirroring those observed at the reference site. Temperature and VPD positively affect both isotopes, while water table and precipitation show inverse relationships. Among the hydroclimate variables, water table, precipitation, and vapor pressure deficit emerge as the primary drivers across all sites. Although δ13C show slightly stronger response to hydroclimate conditions, δ18O displays a more uniform pattern, with stronger responses at the peatland sites compared to the reference site. Moreover, the temporal stability of the responses, although significant for both isotope ratios throughout the entire analysed period, is more pronounced for δ18O. The weak and inconsistent responses in TRW hinder the identification of limiting growth factors. Thus, our findings underscore the value of peatland tree rings and highlight the superiority of δ13C and δ18O over TRW in dendroclimatological research conducted within such environments.
The strong responses observed in living peatland trees suggest the potential for using stable isotopes from subfossil peatland trees to reconstruct past hydroclimatic changes, a task not achievable so far using TRW. However, merging δ13C or δ18O data from different sites, or combining δ13C with δ18O data from the same site to enhance the climate signal, requires careful consideration of multi-decadal/long-term trends to avoid blending different low-frequency trends which could introduce long-term artifacts in hydroclimate reconstruction.
The raw data supporting the conclusions of this article will be made available by the corresponding author Karolina Janecka (karolina.janecka@wsl.ch), without undue reservation.
The supplement related to this article is available online at https://doi.org/10.5194/cp-21-1679-2025-supplement.
KJ, SP, LF and MS conceived the study. MS, CC and JE received the funding for the study. KJ, SP, LF, JE, and MAN collected samples. KJ and MAN performed labwork. KJ analysed the data with input from KT. KJ wrote the manuscript with input from KT. VL provided water table data. SP, LF, MAN, JE, CC, VL, MS reviewed and edited the manuscript. All authors contributed to the discussion of the manuscript and approved the submitted version.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Also, please note that this paper has not received English language copy-editing. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
The SITES and ICOS-Sweden research infrastructures are thankful for providing site-specific data from the Mycklemossen and for the accommodation during fieldwork campaigns within the Skogaryd Research Catchment.
This study was an integrated part of the TURBERAS and MOSS projects. TURBERAS: Reconstruction of Holocene hydro-climatic fluctuations based on multi-proxy peatland records (Swiss National Science Foundation, grant no. 200021_182032). MOSS – Management strategies for tree colonized peatland ecosystems (Svenska Forskningsrådet Formas, FORMAS grant no. 2020-00936). Kerstin Treydte has been supported by the Swiss National Science Foundation (grant no. 175888).
This paper was edited by Julie Loisel and reviewed by two anonymous referees.
Ballesteros-Cánovas, J. A., Edvardsson, J., Corona, C., Mažeika, J., and Stoffel, M.: Estimation of recent peat accumulation with tree saplings, Progress in Physical Geography: Earth and Environment, 46, 515–529, https://doi.org/10.1177/03091333211073786, 2022.
Barbour, M. M., Roden, J. S., Farquhar, G. D., and Ehleringer, J. R.: Expressing leaf water and cellulose oxygen isotope ratios as enrichment above source water reveals evidence of a Péclet effect, Oecologia, 138, 426–435, https://doi.org/10.1007/s00442-003-1449-3, 2004.
Becker, T., Kutzbach, L., Forbrich, I., Schneider, J., Jager, D., Thees, B., and Wilmking, M.: Do we miss the hot spots? – The use of very high resolution aerial photographs to quantify carbon fluxes in peatlands, Biogeosciences, 5, 1387–1393, https://doi.org/10.5194/bg-5-1387-2008, 2008.
Bégin, C., Gingras, M., Savard, M. M., Marion, J., Nicault, A., and Bégin, Y.: Assessing tree-ring carbon and oxygen stable isotopes for climate reconstruction in the Canadian northeastern boreal forest, Palaeogeogr. Palaeocl., 423, 91–101, https://doi.org/10.1016/j.palaeo.2015.01.021, 2015.
Belmecheri, S. and Lavergne, A.: Compiled records of atmospheric CO2 concentrations and stable carbon isotopes to reconstruct climate and derive plant ecophysiological indices from tree rings, Dendrochronologia, 63, 125748, https://doi.org/10.1016/j.dendro.2020.125748, 2020.
Boettger, T., Haupt, M., Knöller, K., Weise, S. M., Waterhouse, J. S., Rinne, K. T., Loader, N. J., Sonninen, E., Jungner, H., Masson-Delmotte, V., Stievenard, M., Guillemin, M.-T., Pierre, M., Pazdur, A., Leuenberger, M., Filot, M., Saurer, M., Reynolds, C. E., Helle, G., and Schleser, G. H.: Wood Cellulose Preparation Methods and Mass Spectrometric Analyses of δ13C, δ18O, and Nonexchangeable δ2H Values in Cellulose, Sugar, and Starch: An Interlaboratory Comparison, Anal. Chem., 79, 4603–4612, https://doi.org/10.1021/ac0700023, 2007.
Boggie, R.: Effect of Water-Table Height on Root Development of Pinus Contorta on Deep Peat in Scotland, Oikos, 23, 304, https://doi.org/10.2307/3543168, 1972.
Briffa, K. R., Osborn, T. J., Schweingruber, F. H., Jones, P. D., Shiyatov, S. G., and Vaganov, E. A.: Tree-ring width and density data around the Northern Hemisphere: Part 1, local and regional climate signals, The Holocene, 12, 737–757, https://doi.org/10.1191/0959683602hl587rp, 2002.
Bunn, A., Korpela, M., Biondi, F., Merian, P., Qeadan, F., and Zang, C.: dplR: Dendrochronology Program Library in R, R package version 1.7.6, https://CRAN.R-project.org/package=dplR (last access: 11 June 2025), 2012.
Büntgen, U., Urban, O., Krusic, P. J., Rybníček, M., Kolář, T., Kyncl, T., Ač, A., Koňasová, E., Čáslavský, J., Esper, J., Wagner, S., Saurer, M., Tegel, W., Dobrovolný, P., Cherubini, P., Reinig, F., and Trnka, M.: Recent European drought extremes beyond Common Era background variability, Nat. Geosci., 14, 190–196, https://doi.org/10.1038/s41561-021-00698-0, 2021.
Carrer, M.: Individualistic and Time-Varying Tree-Ring Growth to Climate Sensitivity, PLoS ONE, 6, e22813, https://doi.org/10.1371/journal.pone.0022813, 2011.
Cedro, A. and Lamentowicz, M.: Contrasting responses to environmental changes by pine (Pinus sylvestris L.) growing on peat and mineral soil: An example from a Polish Baltic bog, Dendrochronologia, 29, 211–217, https://doi.org/10.1016/j.dendro.2010.12.004, 2011.
Cernusak, L. A. and English, N. B.: Beyond tree-ring widths: stable isotopes sharpen the focus on climate responses of temperate forest trees, Tree Physiol., 35, 1–3, https://doi.org/10.1093/treephys/tpu115, 2015.
Chambers, F. M. and Charman, D. J.: Holocene environmental change: contributions from the peatland archive, The Holocene, 14, 1–6, https://doi.org/10.1191/0959683604hl684ed, 2004.
Charman, D., Brown, A., Hendon, D., and Karofeld, E.: Testing the relationship between Holocene peatland palaeoclimate reconstructions and instrumental data at two European sites, Quaternary Sci. Rev., 23, 137–143, https://doi.org/10.1016/j.quascirev.2003.10.006, 2004.
Cook, E. R. and Peters, K.: The Smoothing Spline: A New Approach to Standardizing Forest Interior Tree-Ring Width Series for Dendroclimatic Studies, Tree-Ring Bulletin, 41, 45–53, 1981.
Cuny, H. E., Rathgeber, C. B. K., Frank, D., Fonti, P., Mäkinen, H., Prislan, P., Rossi, S., Del Castillo, E. M., Campelo, F., Vavrčík, H., Camarero, J. J., Bryukhanova, M. V., Jyske, T., Gričar, J., Gryc, V., De Luis, M., Vieira, J., Čufar, K., Kirdyanov, A. V., Oberhuber, W., Treml, V., Huang, J.-G., Li, X., Swidrak, I., Deslauriers, A., Liang, E., Nöjd, P., Gruber, A., Nabais, C., Morin, H., Krause, C., King, G., and Fournier, M.: Woody biomass production lags stem-girth increase by over one month in coniferous forests, Nat. Plants, 1, 15160, https://doi.org/10.1038/nplants.2015.160, 2015.
Dahlke, H. E. and Lyon, S. W.: Early melt season snowpack isotopic evolution in the Tarfala valley, northern Sweden, Ann. Glaciol., 54, 149–156, https://doi.org/10.3189/2013AoG62A232, 2013.
Dey, P.: Estimation of Potential Evapotranspiration using Mcguinnes Bordne formulation, MATLAB Central File Exchange, https://www.mathworks.com/matlabcentral/ fileexchange/65593-estimation-of-potential-evapotranspiration-using-mcguinnes-bordne-formulation (last access: 16 April 2024), 2024.
Dinella, A., Giammarchi, F., and Tonon, G.: Are living peatland trees a reliable natural archive for climate reconstruction?, IAWA J., 40, 366–379, https://doi.org/10.1163/22941932-40190228, 2019.
Eckstein, J., Leuschner, H. H., Bauerochse, A., and Sass-Klaassen, U.: Subfossil bog-pine horizons document climate and ecosystem changes during the Mid-Holocene, Dendrochronologia, 27, 129–146, https://doi.org/10.1016/j.dendro.2009.06.007, 2009.
Edvardsson, J. and Hansson, A.: Multiannual hydrological responses in Scots pine radial growth within raised bogs in southern Sweden, Silva Fenn., 49, 1354, https://doi.org/10.14214/sf.1354, 2015.
Edvardsson, J., Leuschner, H. H., Linderson, H., Linderholm, H. W., and Hammarlund, D.: South Swedish bog pines as indicators of Mid-Holocene climate variability, Dendrochronologia, 30, 93–103, https://doi.org/10.1016/j.dendro.2011.02.003, 2012a.
Edvardsson, J., Linderson, H., Rundgren, M., and Hammarlund, D.: Holocene peatland development and hydrological variability inferred from bog-pine dendrochronology and peat stratigraphy – a case study from southern Sweden, J. Quaternary Sci., 27, 553–563, https://doi.org/10.1002/jqs.2543, 2012b.
Edvardsson, J., Edwards, T. W., Linderson, H., and Hammarlund, D.: Exploring climate forcing of growth depression in subfossil South Swedish bog pines using stable isotopes, Dendrochronologia, 32, 55–61, https://doi.org/10.1016/j.dendro.2013.08.002, 2014.
Edvardsson, J., Rimkus, E., Corona, C., Šimanauskienė, R., Kažys, J., and Stoffel, M.: Exploring the impact of regional climate and local hydrology on Pinus sylvestris L. growth variability – A comparison between pine populations growing on peat soils and mineral soils in Lithuania, Plant Soil, 392, 345–356, https://doi.org/10.1007/s11104-015-2466-9, 2015a.
Edvardsson, J., Šimanauskienė, R., Taminskas, J., Baužienė, I., and Stoffel, M.: Increased tree establishment in Lithuanian peat bogs – Insights from field and remotely sensed approaches, Sci. Total Environ., 505, 113–120, https://doi.org/10.1016/j.scitotenv.2014.09.078, 2015b.
Edvardsson, J., Stoffel, M., Corona, C., Bragazza, L., Leuschner, H. H., Charman, D. J., and Helama, S.: Subfossil peatland trees as proxies for Holocene palaeohydrology and palaeoclimate, Earth-Sci. Rev., 163, 118–140, https://doi.org/10.1016/j.earscirev.2016.10.005, 2016.
Ehleringer, J. R. and Dawson, T. E.: Water uptake by plants: perspectives from stable isotope composition, Plant Cell Environ., 15, 1073–1082, 1992.
Esper, J., Holzkämper, S., Büntgen, U., Schöne, B., Keppler, F., Hartl, C., George, S. St., Riechelmann, D. F. C., and Treydte, K.: Site-specific climatic signals in stable isotope records from Swedish pine forests, Trees, 32, 855–869, https://doi.org/10.1007/s00468-018-1678-z, 2018.
Farquhar, G. D., Ehleringer, J., and Hubick, K.: Carbon isotope discrimination and photosynthesis, Annu. Rev. Plant Biol., 40, 503–537, https://doi.org/10.1146/annurev.pp.40.060189.002443, 1989.
Fonti, P., Heller, O., Cherubini, P., Rigling, A., and Arend, M.: Wood anatomical responses of oak saplings exposed to air warming and soil drought, Plant Biol., 15, 210–219, https://doi.org/10.1111/j.1438-8677.2012.00599.x, 2013.
Francon, L., Edvardsson, J., Corona, C., and Stoffel, M.: The timing of wood formation in peatland trees as obtained with different approaches, Dendrochronologia, 85, 126210, https://doi.org/10.1016/j.dendro.2024.126210, 2024.
Freléchoux, F., Buttler, A., Schweingruber, F. H., and Gobat, J. M.: Stand structure, invasion, and growth dynamics of bog pine (Pinus uncinata var. rotundata) in relation to peat cutting and drainage in the Jura Mountains, Switzerland, Can. J. Forest Res., 30, 1114–1124, 2000.
Fritts, H. C.: Tree rings and climate, 2. print., Academic Press, London, 567 pp., ISBN 012268450-8, 1978.
Gärtner, H. and Nievergelt, D.: The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters, Dendrochronologia, 28, 85–92, https://doi.org/10.1016/j.dendro.2009.09.002, 2010.
Gessler, A., Brandes, E., Keitel, C., Boda, S., Kayler, Z. E., Granier, A., Barbour, M., Farquhar, G. D., and Treydte, K.: The oxygen isotope enrichment of leaf-exported assimilates – does it always reflect lamina leaf water enrichment?, New Phytol., 200, 144–157, https://doi.org/10.1111/nph.12359, 2013.
Gessler, A., Ferrio, J. P., Hommel, R., Treydte, K., Werner, R. A., and Monson, R. K.: Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood, Tree Physiol., 34, 796–818, https://doi.org/10.1093/treephys/tpu040, 2014.
Gore, A. J. P. and Goodall, D. W.: Ecosystems of the world: swamp, bog, fen and moor, Elsevier Scientific Publ, Amsterdam Oxford New York, ISBN 0-444-42003-7, 1983.
Gorham, E.: Northern Peatlands: Role in the Carbon Cycle and Probable Responses to Climatic Warming, Ecol. Appl., 1, 182–195, https://doi.org/10.2307/1941811, 1991.
Harris, I., Osborn, T. J., Jones, P., and Lister, D.: Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset, Sci. Data, 7, 109, https://doi.org/10.1038/s41597-020-0453-3, 2020.
Hartl-Meier, C., Zang, C., Buntgen, U., Esper, J., Rothe, A., Gottlein, A., Dirnbock, T., and Treydte, K.: Uniform climate sensitivity in tree-ring stable isotopes across species and sites in a mid-latitude temperate forest, Tree Physiol., 35, 4–15, https://doi.org/10.1093/treephys/tpu096, 2015.
He, W., Mäkiranta, P., Straková, P., Ojanen, P., Penttilä, T., Bhuiyan, R., Minkkinen, K., and Laiho, R.: Fine-root production in boreal peatland forests: Effects of stand and environmental factors, Forest Ecol. Manag., 550, 121503, https://doi.org/10.1016/j.foreco.2023.121503, 2023.
He, W., Mäkiranta, P., Ojanen, P., Korrensalo, A., and Laiho, R.: Dynamics of fine-root decomposition and its response to site nutrient regimes in boreal drained-peatland and mineral-soil forests, Forest Ecol. Manag., 582, 122564, https://doi.org/10.1016/j.foreco.2025.122564, 2025.
Heikurainen, L.: Structure of Scots pine root systems in a pine swamp and effect of draining on the structure, Acta Forestalia Fennica, 65, 7466, https://doi.org/10.14214/aff.7466, 1955.
Helama, S., Arppe, L., Timonen, M., Mielikäinen, K., and Oinonen, M.: Age-related trends in subfossil tree-ring δ13C data, Chem. Geol., 416, 28–35, https://doi.org/10.1016/j.chemgeo.2015.10.019, 2015.
Hilasvuori, E., Berninger, F., Sonninen, E., Tuomenvirta, H., and Jungner, H.: Stability of climate signal in carbon and oxygen isotope records and ring width from Scots pine (Pinus sylvestris L.) in Finland, J. Quaternary Sci., 24, 469–480, https://doi.org/10.1002/jqs.1260, 2009.
Hökkä, H., Salminen, H., and Ahti, E.: Effect of temperature and precipitation on the annual diameter growth of Scots pine on drained peatlands and adjacent mineral soil sites in Finland, Dendrochronologia, 30, 157–165, https://doi.org/10.1016/j.dendro.2011.02.004, 2012.
Holmes, R. L., Adams, R. K., and Fritts, H. C.: Tree-Ring Chronologies of Western North America: California, Eastern Oregon and Northern Great Basin with Procedures Used in the Chronology Development Work Including Users Manuals for Computer Programs COFECHA and ARSTAN, Laboratory of Tree-Ring Research, University of Arizona, Tucson, AZ, 1986.
Holmgren, M., Lin, C., Murillo, J. E., Nieuwenhuis, A., Penninkhof, J., Sanders, N., Van Bart, T., Van Veen, H., Vasander, H., Vollebregt, M. E., and Limpens, J.: Positive shrub–tree interactions facilitate woody encroachment in boreal peatlands, J. Ecol., 103, 58–66, https://doi.org/10.1111/1365-2745.12331, 2015.
Howie, S. A. and Meerveld, I. T.: The Essential Role of the Lagg in Raised Bog Function and Restoration: A Review, Wetlands, 31, 613–622, https://doi.org/10.1007/s13157-011-0168-5, 2011.
Janecka, K., Kaczka, R. J., Gärtner, H., Harvey, J. E., and Treydte, K.: Compression wood has a minor effect on the climate signal in tree-ring stable isotope records of montane Norway spruce, Tree Physiol., 40, 1014–1028, https://doi.org/10.1093/treephys/tpaa038, 2020.
Joelsson, L. M. T., Engström, E., and Kjellström, E.: Homogenization of Swedish mean monthly temperature series 1860–2021, Int. J. Climatol., 43, 1079–1093, https://doi.org/10.1002/joc.7881, 2023.
Kelly, J., Kljun, N., Eklundh, L., Klemedtsson, L., Liljebladh, B., Olsson, P.-O., Weslien, P., and Xie, X.: Modelling and upscaling ecosystem respiration using thermal cameras and UAVs: Application to a peatland during and after a hot drought, Agr. Forest Meteorol., 300, 108330, https://doi.org/10.1016/j.agrformet.2021.108330, 2021.
Kilian, M. R., Van Der Plicht, J., and Van Geel, B.: Dating raised bogs: New aspects of AMS 14C wiggle matching, a reservoir effect and climatic change, Quaternary Sci. Rev., 14, 959–966, https://doi.org/10.1016/0277-3791(95)00081-X, 1995.
Klesse, S., Weigt, R., Treydte, K., Saurer, M., Schmid, L., Siegwolf, R. T. W., and Frank, D. C.: Oxygen isotopes in tree rings are less sensitive to changes in tree size and relative canopy position than carbon isotopes, Plant Cell Environ., 41, 2899–2914, https://doi.org/10.1111/pce.13424, 2018.
Kozlowski, T. T.: Responses of woody plants to flooding and salinity, Tree physiology, 17, 490–490, 1997.
Kozlowski, T. T.: Plant responses to flooding of soil, BioScience, 34, 162–167, 1984.
Lamentowicz, M., Milecka, K., Gałka, M., Cedro, A., Pawlyta, J., Piotrowska, N., Lamentowicz, Ł., and Van Der Knaap, W. O.: Climate and human induced hydrological change since AD 800 in an ombrotrophic mire in Pomerania (N Poland) tracked by testate amoebae, macro-fossils, pollen and tree rings of pine, Boreas, 38, 214–229, https://doi.org/10.1111/j.1502-3885.2008.00047.x, 2009.
Larsson, L.: CooRecorder and Cdendro programs of the CooRecorder/Cdendro package, http://www.cybis.se/forfun/dendro/, 2013.
Laumer, W., Andreu, L., Helle, G., Schleser, G. H., Wieloch, T., and Wissel, H.: A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses, Rapid Commun. Mass Sp., 40, 1934–1940, https://doi.org/10.1002/rcm.4105, 2009.
Leavitt, S. W.: Seasonal changes in tree rings: species and site coherence, and a possible drought influence, Can. J. Forest Res., 23, 210–218, https://doi.org/10.1139/x93-028, 1993.
Lehsten, D., Von Asmuth, J. R., and Kleyer, M.: Simulation of Water Level Fluctuations in Kettle Holes Using a Time Series Model, Wetlands, 31, 511–520, https://doi.org/10.1007/s13157-011-0174-7, 2011.
Li, X., Chang, S. X., and Salifu, K. F.: Soil texture and layering effects on water and salt dynamics in the presence of a water table: a review, Environ. Rev., 22, 41–50, https://doi.org/10.1139/er-2013-0035, 2014.
Linderholm, H. W.: Climatic Influence on Scots Pine Growth on Dry and Wet Soils in the Central Scandinavian Mountains, Interpreted from Tree-Ring Widths, Silva Fenn., 35, 415–424, 2001.
Linderholm, H. W., Moberg, A., and Grudd, H.: Peatland pines as climate indicators? A regional comparison of the climatic influence on Scots pine growth in Sweden, Can. J. Forest Res., 32, 1400–1410, https://doi.org/10.1139/x02-071, 2002.
Loader, N. J., Santillo, P. M., Woodman-Ralph, J. P., Rolfe, J. E., Hall, M. A., Gagen, M., Robertson, I., Wilson, R., Froyd, C. A., and McCarroll, D.: Multiple stable isotopes from oak trees in southwestern Scotland and the potential for stable isotope dendroclimatology in maritime climatic regions, Chem. Geol., 252, 62–71, https://doi.org/10.1016/j.chemgeo.2008.01.006, 2008.
Malinen, J., Maltamo, N., and Verkasalo, E.: Stem and wood properties of Norway spruce on drained peatlands and mineral forest lands in Southern Finland, Balt. For., 11, 21–37, 2005.
Martínez-Sancho, E., Cernusak, L. A., Fonti, P., Gregori, A., Ullrich, B., Pannatier, E. G., Gessler, A., Lehmann, M. M., Saurer, M., and Treydte, K.: Unenriched xylem water contribution during cellulose synthesis influenced by atmospheric demand governs the intra-annual tree-ring δ18O signature, New Phytol., 240, 1743–1757, https://doi.org/10.1111/nph.19278, 2023.
McCarroll, D. and Loader, N. J.: Stable isotopes in tree rings, Quaternary Sci. Rev., 23, 771–801, https://doi.org/10.1016/j.quascirev.2003.06.017, 2004.
Migoń, P. and Lidmar-Bergström, K.: Weathering mantles and their significance for geomorphological evolution of central and northern Europe since the Mesozoic, Earth-Sci. Rev., 56, 285–324, https://doi.org/10.1016/S0012-8252(01)00068-X, 2001.
Murray, F.: On the computation of saturation vapor pressure, J. Appl. Meteorol., 6, 203–204, https://doi.org/10.1175/1520-0450(1967)006<0203:OTCOSV>2.0.CO;2, 1967.
Nardini, A., Tomasella, M., and Di Bert, S.: Bedrock: the hidden water reservoir for trees challenged by drought, Trees, 38, 1–11, https://doi.org/10.1007/s00468-023-02482-6, 2024.
Offermann, C., Ferrio, J. P., Holst, J., Grote, R., Siegwolf, R., Kayler, Z., and Gessler, A.: The long way down–are carbon and oxygen isotope signals in the tree ring uncoupled from canopy physiological processes?, Tree Physiol., 31, 1088–1102, https://doi.org/10.1093/treephys/tpr093, 2011.
Penttilä, T.: Growth response of peatland stands to drainage in northern Finland, in: Peat and Peatlands – Diversification and Innovation, Canadian Society for Peat and Peatlands 1, 70–77, 1991.
Pilcher, J. R., Baillie, M. G. L., Brown, D. M., McCormac, F. G., Macsweeney, P. B., and McLawrence, A. S.: Dendrochronology of Subfossil Pine in the North of Ireland, J. Ecol., 83, 665, https://doi.org/10.2307/2261634, 1995.
Pouliot, R., Rochefort, L., and Karofeld, E.: Initiation of microtopography in revegetated cutover peatlands: Initiation of microtopography, Appl. Veg. Sci., 14, 158–171, https://doi.org/10.1111/j.1654-109X.2010.01118.x, 2011.
Reynolds-Henne, C. E., Siegwolf, R. T. W., Treydte, K. S., Esper, J., Henne, S., and Saure, M.: Temporal stability of climate-isotope relationships in tree rings of oak and pine (Ticino, Switzerland), Global Biogeochem. Cy., 21, GB4009, https://doi.org/10.1029/2007GB002945, 2007.
Roden, J. S., Lin, G., and Ehleringer, J. R.: A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in tree-ring cellulose, Geochim. Cosmochim. Ac., 64, 21–35, https://doi.org/10.1016/S0016-7037(99)00195-7, 2000.
Rozanski, K., Araguas-Araguas, L., and Gonfiantini, R.: Relation between long-term trends of oxygen-18 isotope composition of precipitation and climate, Science, 258, 981–985, 1992.
Sarris, D., Siegwolf, R., and Körner, C.: Inter- and intra-annual stable carbon and oxygen isotope signals in response to drought in Mediterranean pines, Agr. Forest Meteorol., 168, 59–68, https://doi.org/10.1016/j.agrformet.2012.08.007, 2013.
Saurer, M., Siegenthaler, U., and Schweingruber, F.: The climate-carbon isotope relationship in tree rings and the significance of site conditions, Tellus B, 47, 320–330, https://doi.org/10.3402/tellusb.v47i3.16051, 1995.
Saurer, M., Siegwolf, R. T. W., and Schweingruber, F. H.: Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years, Glob. Change Biol., 10, 2109–2120, https://doi.org/10.1111/j.1365-2486.2004.00869.x, 2004.
Saurer, M., Cherubini, P., Reynolds-Henne, C. E., Treydte, K. S., Anderson, W. T., and Siegwolf, R. T. W.: An investigation of the common signal in tree ring stable isotope chronologies at temperate sites, J. Geophys. Res., 113, G04035, https://doi.org/10.1029/2008JG000689, 2008.
Saurer, M., Spahni, R., Frank, D. C., Joos, F., Leuenberger, M., Loader, N. J., McCarroll, D., Gagen, M., Poulter, B., Siegwolf, R. T. W., Andreu-Hayles, L., Boettger, T., Dorado Liñán, I., Fairchild, I. J., Friedrich, M., Gutierrez, E., Haupt, M., Hilasvuori, E., Heinrich, I., Helle, G., Grudd, H., Jalkanen, R., Levanič, T., Linderholm, H. W., Robertson, I., Sonninen, E., Treydte, K., Waterhouse, J. S., Woodley, E. J., Wynn, P. M., and Young, G. H. F.: Spatial variability and temporal trends in water-use efficiency of European forests, Glob. Change Biol., 20, 3700–3712, https://doi.org/10.1111/gcb.12717, 2014.
Seftigen, K., Linderholm, H. W., Loader, N. J., Liu, Y., and Young, G. H. F.: The influence of climate on and ratios in tree ring cellulose of Pinus sylvestris L. growing in the central Scandinavian Mountains, Chem. Geol., 286, 84–93, https://doi.org/10.1016/j.chemgeo.2011.04.006, 2011.
Siegwolf, R. T. W., Brooks, J. R., Roden, J., and Saurer, M. (Eds.): Stable Isotopes in Tree Rings: Inferring Physiological, Climatic and Environmental Responses, Springer International Publishing, Cham, https://doi.org/10.1007/978-3-030-92698-4, 2022.
Smiljanić, M. and Wilmking, M.: Drivers of stem radial variation and its pattern in peatland Scots pines: A pilot study, Dendrochronologia, 47, 30–37, https://doi.org/10.1016/j.dendro.2017.12.001, 2018.
Smiljanić, M., Seo, J.-W., Läänelaid, A., Van Der Maaten-Theunissen, M., Stajić, B., and Wilmking, M.: Peatland pines as a proxy for water table fluctuations: Disentangling tree growth, hydrology and possible human influence, Sci. Total Environ., 500–501, 52–63, https://doi.org/10.1016/j.scitotenv.2014.08.056, 2014.
Timell, T. E.: Compression wood in gymnosperms, Springer-Verlag, Berlin Heidelberg New York Tokyo, ISBN 978-3-540-15715-1, 1986.
Torbenson, M., Klippel, L., Hartl, C., Reinig, F., Treydte, K., Büntgen, U., Trnka, M., Schöne, B., Schneider, L., and Esper, J.: Investigation of age trends in tree-ring stable carbon and oxygen isotopes from northern Fennoscandia over the past millennium, Quatern. Int., 631, 105–114, https://doi.org/10.1016/j.quaint.2022.05.017, 2022.
Treydte, K., Schleser, G. H., Schweingruber, F. H., and Winiger, M.: The climatic significance of δ13C in subalpine spruces (Lötschental, Swiss Alps): A case study with respect to altitude, exposure and soil moisture, Tellus B, 53, 593–611, https://doi.org/10.3402/tellusb.v53i5.16639, 2001.
Treydte, K., Frank, D., Esper, J., Andreu, L., Bednarz, Z., Berninger, F., Boettger, T., D'Alessandro, C. M., Etien, N., Filot, M., Grabner, M., Guillemin, M. T., Gutierrez, E., Haupt, M., Helle, G., Hilasvuori, E., Jungner, H., Kalela-Brundin, M., Krapiec, M., Leuenberger, M., Loader, N. J., Masson-Delmotte, V., Pazdur, A., Pawelczyk, S., Pierre, M., Planells, O., Pukiene, R., Reynolds-Henne, C. E., Rinne, K. T., Saracino, A., Saurer, M., Sonninen, E., Stievenard, M., Switsur, V. R., Szczepanek, M., Szychowska-Krapiec, E., Todaro, L., Waterhouse, J. S., Weigl, M., and Schleser, G. H.: Signal strength and climate calibration of a European tree-ring isotope network, Geophys. Res. Lett., 34, L24302, https://doi.org/10.1029/2007GL031106, 2007.
Treydte, K., Boda, S., Graf Pannatier, E., Fonti, P., Frank, D., Ullrich, B., Saurer, M., Siegwolf, R., Battipaglia, G., Werner, W., and Gessler, A.: Seasonal transfer of oxygen isotopes from precipitation and soil to the tree ring: source water versus needle water enrichment, New Phytol., 202, 772–783, https://doi.org/10.1111/nph.12741, 2014.
Treydte, K., Liu, L., Padrón, R. S., Martínez-Sancho, E., Babst, F., Frank, D. C., Gessler, A., Kahmen, A., Poulter, B., Seneviratne, S. I., Stegehuis, A. I., Wilson, R., Andreu-Hayles, L., Bale, R., Bednarz, Z., Boettger, T., Berninger, F., Büntgen, U., Daux, V., Dorado-Liñán, I., Esper, J., Friedrich, M., Gagen, M., Grabner, M., Grudd, H., Gunnarsson, B. E., Gutiérrez, E., Hafner, P., Haupt, M., Hilasvuori, E., Heinrich, I., Helle, G., Jalkanen, R., Jungner, H., Kalela-Brundin, M., Kessler, A., Kirchhefer, A., Klesse, S., Krapiec, M., Levanič, T., Leuenberger, M., Linderholm, H. W., McCarroll, D., Masson-Delmotte, V., Pawelczyk, S., Pazdur, A., Planells, O., Pukiene, R., Rinne-Garmston, K. T., Robertson, I., Saracino, A., Saurer, M., Schleser, G. H., Seftigen, K., Siegwolf, R. T. W., Sonninen, E., Stievenard, M., Szychowska-Krapiec, E., Szymaszek, M., Todaro, L., Waterhouse, J. S., Weigl-Kuska, M., Weigt, R. B., Wimmer, R., Woodley, E. J., Vitas, A., Young, G., and Loader, N. J.: Recent human-induced atmospheric drying across Europe unprecedented in the last 400 years, Nat. Geosci., 17, 58–65, https://doi.org/10.1038/s41561-023-01335-8, 2024.
Treydte, K. S., Frank, D. C., Saurer, M., Helle, G., Schleser, G. H., and Esper, J.: Impact of climate and CO2 on a millennium-long tree-ring carbon isotope record, Geochim. Cosmochim. Ac., 73, 4635–4647, https://doi.org/10.1016/j.gca.2009.05.057, 2009.
Vitas, A. and Erlickytë, R.: Influence of droughts to the radial growth of Scots Pine (Pinus sylvestris L.) at different site conditions, Balt. For., 13, 10–16, 2007.
Von Asmuth, J. R., Bierkens, M. F. P., and Maas, K.: Transfer function-noise modeling in continuous time using predefined impulse response functions, Water Resources Research, 38, 23-1–23-12, https://doi.org/10.1029/2001WR001136, 2002.
Von Asmuth, J. R., Maas, K., Bakker, M., and Petersen, J.: Modeling Time Series of Ground Water Head Fluctuations Subjected to Multiple Stresses, Groundwater, 46, 30–40, https://doi.org/10.1111/j.1745-6584.2007.00382.x, 2008.
White, J. D., Ahrén, D., Ström, L., Kelly, J., Klemedtsson, L., Keane, B., and Parmentier, F.-J. W.: Methane producing and reducing microorganisms display a high resilience to drought in a Swedish hemi-boreal mire, ESS Open Archive [preprint], https://doi.org/10.22541/essoar.167327310.06866368/v1, 2023.
Wigley, T., Briffa, K. R., and Jones, P.: On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology, J. Appl. Meteorol. Clim., 23, 201–2013, 1984.
Wilmking, M., Hallinger, M., Van Bogaert, R., Kyncl, T., Babst, F., Hahne, W., Juday, G. P., De Luis, M., Novak, K., and Völlm, C.: Continuously missing outer rings in woody plants at their distributional margins, Dendrochronologia, 30, 213–222, https://doi.org/10.1016/j.dendro.2011.10.001, 2012.
Wilmking, M., Van Der Maaten-Theunissen, M., Van Der Maaten, E., Scharnweber, T., Buras, A., Biermann, C., Gurskaya, M., Hallinger, M., Lange, J., Shetti, R., Smiljanic, M., and Trouillier, M.: Global assessment of relationships between climate and tree growth, Glob. Change Biol., 26, 3212–3220, https://doi.org/10.1111/gcb.15057, 2020.
Young, G. H. F., Demmler, J. C., Gunnarson, B. E., Kirchhefer, A. J., Loader, N. J., and McCarroll, D.: Age trends in tree ring growth and isotopic archives: A case study of Pinus sylvestris L. from northwestern Norway, Global Biogeochem. Cy., 25, GB2020, https://doi.org/10.1029/2010GB003913, 2011.
Zang, C. and Biondi, F.: treeclim: an R package for the numerical calibration of proxy-climate relationships, Ecography, 38, 431–436, https://doi.org/10.1111/ecog.01335, 2015.
Zeiger, E. and Farquhar, G. D. (Eds.): Stomatal function, Stanford University Press, Stanford, CA, 503 pp., ISBN 0-8047-1347-2, 1987.
Zoltai, S. C. and Pettapiece, W. W.: Tree distribution on perennially frozen earth hummocks, Arctic Alpine Res., 6, 403–411, 1974.